The effect of gentamicin-induced readthrough on a novel premature termination codon of CD18 leukocyte adhesion deficiency patients
- PMID: 21103413
- PMCID: PMC2982813
- DOI: 10.1371/journal.pone.0013659
The effect of gentamicin-induced readthrough on a novel premature termination codon of CD18 leukocyte adhesion deficiency patients
Abstract
Background: Leukocyte adhesion deficiency 1 (LAD1) is an inherited disorder of neutrophil function. Nonsense mutations in the affected CD18 (ITB2) gene have rarely been described. In other genes containing such mutations, treatments with aminoglycoside types of antibiotics (e.g., gentamicin) were reported to partially correct the premature protein termination, by induction of readthrough mechanism.
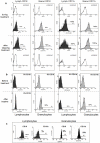
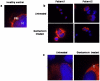
Methodology/principal findings: Genetic analysis was performed on 2 LAD1 patients. Expression, functional and immunofluorescence assays of CD18 in the patients were used to determine the in-vivo and in-vitro effects of gentamicin-induced readthrough. A theoretical modeling of the corrected CD18 protein was developed to predict the protein function.
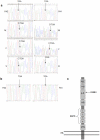
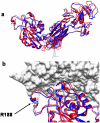
Results: We found a novel premature termination codon, C562T (R188X), in exon 6 of the CD18 gene that caused a severe LAD1 phenotype in two unrelated Palestinian children. In-vivo studies on these patients' cells after gentamicin treatment showed abnormal adhesion and chemotactic functions, while in-vitro studies showed mislocalization of the corrected protein to the cytoplasm and not to the cell surface. A theoretical modeling of the corrected CD18 protein suggested that the replacement of the wild type arginine by gentamicin induced tryptophan at the position of the nonsense mutation, although enabled the expression of the entire CD18 protein, this was not sufficient to stabilize the CD18/11 heterodimer at the cell surface.
Conclusion: A novel nonsense mutation in the CD18 gene causing a complete absence of CD18 protein and severe LAD1 clinical phenotype is reported. Both in vivo and in vitro treatments with gentamicin resulted in the expression of a corrected full-length dysfunctional or mislocalized CD18 protein. However, while the use of gentamicin increased the expression of CD18, it did not improve leukocyte adhesion and chemotaxis. Moreover, the integrity of the CD18/CD11 complex at the cell surface was impaired, due to abnormal CD18 protein and possibly lack of CD11a expression.
Conflict of interest statement
Figures

Similar articles
-
Clinical and Genetic Spectrum of a Large Cohort of Patients With Leukocyte Adhesion Deficiency Type 1 and 3: A Multicentric Study From India.Front Immunol. 2020 Dec 16;11:612703. doi: 10.3389/fimmu.2020.612703. eCollection 2020. Front Immunol. 2020. PMID: 33391282 Free PMC article.
-
Genetic analysis of patients with leukocyte adhesion deficiency: genomic sequencing reveals otherwise undetectable mutations.Exp Hematol. 2002 Mar;30(3):252-61. doi: 10.1016/s0301-472x(01)00782-2. Exp Hematol. 2002. PMID: 11882363
-
The minor gentamicin complex component, X2, is a potent premature stop codon readthrough molecule with therapeutic potential.PLoS One. 2018 Oct 25;13(10):e0206158. doi: 10.1371/journal.pone.0206158. eCollection 2018. PLoS One. 2018. PMID: 30359426 Free PMC article.
-
β2 Integrin Gene (ITGB2) mutation spectra in Pakistani families with leukocyte adhesion deficiency type 1 (LAD1).Immunobiology. 2020 May;225(3):151938. doi: 10.1016/j.imbio.2020.151938. Epub 2020 Apr 2. Immunobiology. 2020. PMID: 32279896 Review.
-
Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response.Immunol Rev. 1990 Apr;114:145-80. doi: 10.1111/j.1600-065x.1990.tb00564.x. Immunol Rev. 1990. PMID: 1973407 Review.
Cited by
-
Highlighting the problematic reliance on CD18 for diagnosing leukocyte adhesion deficiency type 1.Immunol Res. 2016 Apr;64(2):476-82. doi: 10.1007/s12026-015-8706-5. Immunol Res. 2016. PMID: 26434744
-
Synthetic aminoglycosides efficiently suppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor.Am J Respir Cell Mol Biol. 2014 Apr;50(4):805-16. doi: 10.1165/rcmb.2013-0282OC. Am J Respir Cell Mol Biol. 2014. PMID: 24251786 Free PMC article.
-
Lessons learned from phagocytic function studies in a large cohort of patients with recurrent infections.J Clin Immunol. 2012 Jun;32(3):454-66. doi: 10.1007/s10875-011-9633-4. Epub 2011 Dec 30. J Clin Immunol. 2012. PMID: 22207252
-
Therapeutics based on stop codon readthrough.Annu Rev Genomics Hum Genet. 2014;15:371-94. doi: 10.1146/annurev-genom-091212-153527. Epub 2014 Apr 18. Annu Rev Genomics Hum Genet. 2014. PMID: 24773318 Free PMC article. Review.
-
Readthrough Activators and Nonsense-Mediated mRNA Decay Inhibitor Molecules: Real Potential in Many Genetic Diseases Harboring Premature Termination Codons.Pharmaceuticals (Basel). 2024 Feb 28;17(3):314. doi: 10.3390/ph17030314. Pharmaceuticals (Basel). 2024. PMID: 38543100 Free PMC article. Review.
References
-
- Dinauer MC. Disorders of neutrophil function: an overview. Methods Mol Biol. 2007;412:489–504. - PubMed
-
- Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol. 2002;9:30–35. - PubMed
-
- Fiorini M, Piovani G, Schumacher RF, Magri C, Bertini V, et al. ITGB2 mutation combined with deleted ring 21 chromosome in a child with leukocyte adhesion deficiency. J Allergy Clin Immunol. 2009;124:1356–1358. - PubMed
-
- Xi B, Guan F, Lawrence DS. Enhanced production of functional proteins from defective genes. J Am Chem Soc. 2004;126:5660–5661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

