A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine
- PMID: 21088235
- PMCID: PMC3043655
- DOI: 10.1152/ajpgi.00278.2010
A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine
Abstract
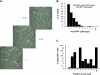
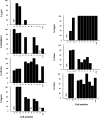
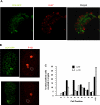
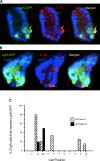
The spatial orientation of the enteroendocrine cells along the crypt-villus axis is closely associated with their differentiation in the intestine. Here we studied this relationship using primary duodenal crypts and an ex vivo organoid system established from cholecystokinin-green fluorescent protein (CCK-GFP) transgenic mice. In the primary duodenal crypts, GFP+ cells were found not only in the upper crypt but also at the crypt base, where the stem cells reside. Many GFP+ cells below +4 position were positive for the putative intestinal stem cell markers, leucine-rich repeat-containing G protein-coupled receptor 5, CD133, and doublecortin and CaM kinase-like-1, and also for the neuroendocrine transcription factor neurogenin 3. However, these cells were neither stem nor transient amplifying precursor cells because they were negative for both Ki-67 and phospho-Histone H3 and positive for the mature endocrine marker chromogranin A. Furthermore, these cells expressed multiple endocrine hormones. Tracking of GFP+ cells in the organoids from CCK-GFP mice indicated that GFP+ cells were first observed around the +4 position, some of which localized to the crypt base later in the culture period. These results suggest that a subset of enteroendocrine cells migrates down to the crypt base or stays localized at the crypt base, where they express stem and postmitotic endocrine markers. Further investigation of the function of this subset may provide novel insights into the genesis and development of enteroendocrine cells as well as enteroendocrine tumorigenesis.
Figures



Similar articles
-
Comparative analysis of enteroendocrine cells and their hormones between mouse intestinal organoids and native tissues.Biosci Biotechnol Biochem. 2020 May;84(5):936-942. doi: 10.1080/09168451.2020.1713043. Epub 2020 Jan 9. Biosci Biotechnol Biochem. 2020. PMID: 31916916
-
The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin.Gastroenterology. 2011 Mar;140(3):903-12. doi: 10.1053/j.gastro.2010.10.012. Epub 2010 Oct 16. Gastroenterology. 2011. PMID: 20955703 Free PMC article.
-
Nkx2.2 is expressed in a subset of enteroendocrine cells with expanded lineage potential.Am J Physiol Gastrointest Liver Physiol. 2015 Dec 15;309(12):G975-87. doi: 10.1152/ajpgi.00244.2015. Epub 2015 Oct 22. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26492922 Free PMC article.
-
The role of SOX9 transcription factor in pancreatic and duodenal development.Stem Cells Dev. 2013 Nov 15;22(22):2935-43. doi: 10.1089/scd.2013.0106. Epub 2013 Aug 2. Stem Cells Dev. 2013. PMID: 23806070 Review.
-
Enteroendocrine Dynamics - New Tools Reveal Hormonal Plasticity in the Gut.Endocr Rev. 2020 Oct 1;41(5):bnaa018. doi: 10.1210/endrev/bnaa018. Endocr Rev. 2020. PMID: 32531023 Free PMC article. Review.
Cited by
-
Reserve Stem Cells in Intestinal Homeostasis and Injury.Gastroenterology. 2018 Nov;155(5):1348-1361. doi: 10.1053/j.gastro.2018.08.016. Epub 2018 Aug 15. Gastroenterology. 2018. PMID: 30118745 Free PMC article. Review.
-
Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation.Am J Physiol Gastrointest Liver Physiol. 2012 May 15;302(10):G1111-32. doi: 10.1152/ajpgi.00519.2011. Epub 2012 Feb 23. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22361729 Free PMC article.
-
Mapping early fate determination in Lgr5+ crypt stem cells using a novel Ki67-RFP allele.EMBO J. 2014 Sep 17;33(18):2057-68. doi: 10.15252/embj.201488017. Epub 2014 Aug 4. EMBO J. 2014. PMID: 25092767 Free PMC article.
-
Subversion of Niche-Signalling Pathways in Colorectal Cancer: What Makes and Breaks the Intestinal Stem Cell.Cancers (Basel). 2021 Feb 27;13(5):1000. doi: 10.3390/cancers13051000. Cancers (Basel). 2021. PMID: 33673710 Free PMC article. Review.
-
The Impact of Di-Isononyl Phthalate Exposure on Specialized Epithelial Cells in the Colon.Toxicol Sci. 2021 Oct 27;184(1):142-153. doi: 10.1093/toxsci/kfab105. Toxicol Sci. 2021. PMID: 34453847 Free PMC article.
References
-
- Aiken KD, Kisslinger JA, Roth KA. Immunohistochemical studies indicate multiple enteroendocrine cell differentiation pathways in the mouse proximal small intestine. Dev Dyn 201: 63– 70, 1994 - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003– 1007, 2007 - PubMed
-
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251– 263, 2002 - PubMed
-
- Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol 300: 722– 735, 2006 - PubMed
-
- Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 160: 77– 91, 1981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

