The role of the transcription factor CREB in immune function
- PMID: 21084670
- PMCID: PMC5519339
- DOI: 10.4049/jimmunol.1001829
The role of the transcription factor CREB in immune function
Abstract
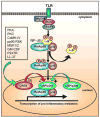
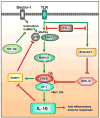
CREB is a transcription factor that regulates diverse cellular responses, including proliferation, survival, and differentiation. CREB is induced by a variety of growth factors and inflammatory signals and subsequently mediates the transcription of genes containing a cAMP-responsive element. Several immune-related genes possess this cAMP-responsive element, including IL-2, IL-6, IL-10, and TNF-α. In addition, phosphorylated CREB has been proposed to directly inhibit NF-κB activation by blocking the binding of CREB binding protein to the NF-κB complex, thereby limiting proinflammatory responses. CREB also induces an antiapoptotic survival signal in monocytes and macrophages. In T and B cells, CREB activation promotes proliferation and survival and differentially regulates Th1, Th2, and Th17 responses. Finally, CREB activation is required for the generation and maintenance of regulatory T cells. This review summarizes current advances involving CREB in immune function--a role that is continually being defined.
Conflict of interest statement
Figures

Similar articles
-
The expanding family of CREB/CREM transcription factors that are involved with spermatogenesis.Mol Cell Endocrinol. 2002 Feb 22;187(1-2):115-24. doi: 10.1016/s0303-7207(01)00696-7. Mol Cell Endocrinol. 2002. PMID: 11988318 Review.
-
PKA and cAMP stimulate proliferation of mouse embryonic stem cells by elevating GLUT1 expression mediated by the NF-κB and CREB/CBP signaling pathways.Biochim Biophys Acta. 2012 Oct;1820(10):1636-46. doi: 10.1016/j.bbagen.2012.05.008. Epub 2012 May 29. Biochim Biophys Acta. 2012. PMID: 22658979
-
Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription.J Immunol. 1997 Dec 1;159(11):5450-6. J Immunol. 1997. PMID: 9548485
-
CREB is one component of the binding complex of the Ces-2/E2A-HLF binding element and is an integral part of the interleukin-3 survival signal.Mol Cell Biol. 2001 Jul;21(14):4636-46. doi: 10.1128/MCB.21.14.4636-4646.2001. Mol Cell Biol. 2001. PMID: 11416141 Free PMC article.
-
The function of cyclic-adenosine monophosphate responsive element-binding protein in hematologic malignancies.Leuk Lymphoma. 2011 Nov;52(11):2057-63. doi: 10.3109/10428194.2011.584994. Leuk Lymphoma. 2011. PMID: 22023600 Review.
Cited by
-
Farrerol Alleviates Cerebral Ischemia-Reperfusion Injury by Promoting Neuronal Survival and Reducing Neuroinflammation.Mol Neurobiol. 2024 Sep;61(9):7239-7255. doi: 10.1007/s12035-024-04031-9. Epub 2024 Feb 20. Mol Neurobiol. 2024. PMID: 38376762
-
Promising drug repurposing approach targeted for cytokine storm implicated in SARS-CoV-2 complications.Immunopharmacol Immunotoxicol. 2021 Aug;43(4):395-409. doi: 10.1080/08923973.2021.1931302. Epub 2021 May 31. Immunopharmacol Immunotoxicol. 2021. PMID: 34057871 Free PMC article. Review.
-
Dietary supplementation with a microencapsulated blend of organic acids and botanicals alters the kinome in the ileum and jejunum of Gallus gallus.PLoS One. 2020 Jul 30;15(7):e0236950. doi: 10.1371/journal.pone.0236950. eCollection 2020. PLoS One. 2020. PMID: 32730335 Free PMC article.
-
Cellular Signaling and Anti-Apoptotic Effects of Prolactin-Releasing Peptide and Its Analog on SH-SY5Y Cells.Int J Mol Sci. 2020 Sep 1;21(17):6343. doi: 10.3390/ijms21176343. Int J Mol Sci. 2020. PMID: 32882929 Free PMC article.
-
Genetics and immunity in the era of single-cell genomics.Hum Mol Genet. 2016 Oct 1;25(R2):R141-R148. doi: 10.1093/hmg/ddw192. Epub 2016 Jul 12. Hum Mol Genet. 2016. PMID: 27412011 Free PMC article. Review.
References
-
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. - PubMed
-
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. - PubMed
-
- Brindle P, Linke S, Montminy M. Protein-kinase-A-dependent activator in transcription factor CREB reveals new role for CREM repressors. Nature. 1993;364:821–824. - PubMed
-
- Enslen H, Tokumitsu H, Soderling TR. Phosphorylation of CREB by CaM-kinase IV activated by CaM-kinase IV kinase. Biochem Biophys Res Commun. 1995;207:1038–1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL075826/HL/NHLBI NIH HHS/United States
- R41 HL078526/HL/NHLBI NIH HHS/United States
- T32 HD07512/HD/NICHD NIH HHS/United States
- R01 HL83077/HL/NHLBI NIH HHS/United States
- T32 HD007512/HD/NICHD NIH HHS/United States
- HL78526/HL/NHLBI NIH HHS/United States
- R01 HL097561/HL/NHLBI NIH HHS/United States
- T32 HD007512-01/HD/NICHD NIH HHS/United States
- R01 AI078910/AI/NIAID NIH HHS/United States
- R01 HL083077/HL/NHLBI NIH HHS/United States
- R03 AR054534/AR/NIAMS NIH HHS/United States
- HL097561/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

