Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny
- PMID: 21084482
- PMCID: PMC3019999
- DOI: 10.1128/JVI.01557-10
Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny
Erratum in
- J Virol. 2013 Jun;87(12):7197
Abstract
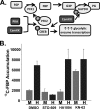
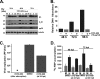
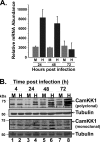
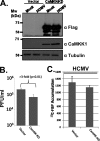
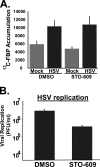
Viruses depend on the host cell to provide the energy and biomolecular subunits necessary for production of viral progeny. We have previously reported that human cytomegalovirus (HCMV) infection induces dramatic changes to central carbon metabolism, including glycolysis, the tricarboxylic acid (TCA) cycle, fatty acid biosynthesis, and nucleotide biosynthesis. Here, we explore the mechanisms involved in HCMV-mediated glycolytic activation. We find that HCMV virion binding and tegument protein delivery are insufficient for HCMV-mediated activation of glycolysis. Viral DNA replication and late-gene expression, however, are not required. To narrow down the list of cellular pathways important for HCMV-mediated [corrected] activation of glycolysis, we utilized pharmaceutical inhibitors to block pathways reported to be both involved in metabolic control and activated by HCMV infection. We find that inhibition of calmodulin-dependent kinase kinase (CaMKK), but not calmodulin-dependent kinase II (CaMKII) or protein kinase A (PKA), blocks HCMV-mediated activation of glycolysis. HCMV infection was also found to target calmodulin-dependent kinase kinase 1 (CaMKK1) expression, increasing the levels of CaMKK1 mRNA and protein. Our results indicate that inhibition of CaMKK has a negligible impact on immediate-early-protein accumulation yet severely attenuates production of HCMV viral progeny, reduces expression of at least one early gene, and blocks viral DNA replication. Inhibition of CaMKK did not affect the glycolytic activation induced by another herpes virus, herpes simplex virus type 1 (HSV-1). Furthermore, inhibition of CaMKK had a much smaller impact on HSV-1 replication than on that of HCMV. These data suggest that the role of CaMKK during the viral life cycle is, in this regard, HCMV specific. Taken together, our results suggest that CaMKK is an important factor for HCMV replication and HCMV-mediated glycolytic activation.
Figures
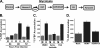

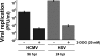
Similar articles
-
Human Cytomegalovirus Induces the Expression of the AMPKa2 Subunit to Drive Glycolytic Activation and Support Productive Viral Infection.J Virol. 2021 Mar 1;95(5):e01321-20. doi: 10.1128/JVI.01321-20. Epub 2020 Dec 2. J Virol. 2021. PMID: 33268515 Free PMC article.
-
HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication.PLoS Pathog. 2012 Jan;8(1):e1002502. doi: 10.1371/journal.ppat.1002502. Epub 2012 Jan 26. PLoS Pathog. 2012. PMID: 22291597 Free PMC article.
-
Human cytomegalovirus induces the activity and expression of acetyl-coenzyme A carboxylase, a fatty acid biosynthetic enzyme whose inhibition attenuates viral replication.J Virol. 2011 Jun;85(12):5814-24. doi: 10.1128/JVI.02630-10. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471234 Free PMC article.
-
Meal for Two: Human Cytomegalovirus-Induced Activation of Cellular Metabolism.Viruses. 2019 Mar 19;11(3):273. doi: 10.3390/v11030273. Viruses. 2019. PMID: 30893762 Free PMC article. Review.
-
Viral effects on metabolism: changes in glucose and glutamine utilization during human cytomegalovirus infection.Trends Microbiol. 2011 Jul;19(7):360-7. doi: 10.1016/j.tim.2011.04.002. Epub 2011 May 12. Trends Microbiol. 2011. PMID: 21570293 Free PMC article. Review.
Cited by
-
Nitric Oxide Circumvents Virus-Mediated Metabolic Regulation during Human Cytomegalovirus Infection.mBio. 2020 Dec 15;11(6):e02630-20. doi: 10.1128/mBio.02630-20. mBio. 2020. PMID: 33323506 Free PMC article.
-
Human Cytomegalovirus Induces the Expression of the AMPKa2 Subunit to Drive Glycolytic Activation and Support Productive Viral Infection.J Virol. 2021 Mar 1;95(5):e01321-20. doi: 10.1128/JVI.01321-20. Epub 2020 Dec 2. J Virol. 2021. PMID: 33268515 Free PMC article.
-
Transcriptome profiling highlights regulated biological processes and type III interferon antiviral responses upon Crimean-Congo hemorrhagic fever virus infection.Virol Sin. 2023 Feb;38(1):34-46. doi: 10.1016/j.virs.2022.09.002. Epub 2022 Sep 6. Virol Sin. 2023. PMID: 36075566 Free PMC article.
-
Cytomegalovirus-induced inactivation of TSC2 disrupts the coupling of fatty acid biosynthesis to glucose availability resulting in a vulnerability to glucose starvation.mBio. 2024 Jan 16;15(1):e0303123. doi: 10.1128/mbio.03031-23. Epub 2023 Dec 20. mBio. 2024. PMID: 38117060 Free PMC article.
-
Hijacking the Supplies: Metabolism as a Novel Facet of Virus-Host Interaction.Front Immunol. 2019 Jul 3;10:1533. doi: 10.3389/fimmu.2019.01533. eCollection 2019. Front Immunol. 2019. PMID: 31333664 Free PMC article. Review.
References
-
- Andrei, G., E. De Clercq, and R. Snoeck. 2008. Novel inhibitors of human CMV. Curr. Opin. Invest. Drugs 9:132-145. - PubMed
-
- Beisser, P. S., H. Lavreysen, C. A. Bruggeman, and C. Vink. 2008. Chemokines and chemokine receptors encoded by cytomegaloviruses. Curr. Top. Microbiol. Immunol. 325:221-242. - PubMed
-
- Bito, H., and S. Takemoto-Kimura. 2003. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium 34:425-430. - PubMed
-
- Bradley, P. L. 1957. Metabolism of pyruvate and alpha-ketoglutarate in virus-infected mouse brain. Nature 180:1418-1419. - PubMed
-
- Burny, W., C. Liesnard, C. Donner, and A. Marchant. 2004. Epidemiology, pathogenesis and prevention of congenital cytomegalovirus infection. Expert Rev. Anti Infect. Ther. 2:881-894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

