Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation
- PMID: 21083385
- PMCID: PMC3148255
- DOI: 10.1056/NEJMoa0909825
Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation
Abstract
Background: A new approach in the treatment of cystic fibrosis involves improving the function of mutant cystic fibrosis transmembrane conductance regulator (CFTR). VX-770, a CFTR potentiator, has been shown to increase the activity of wild-type and defective cell-surface CFTR in vitro.
Methods: We randomly assigned 39 adults with cystic fibrosis and at least one G551D-CFTR allele to receive oral VX-770 every 12 hours at a dose of 25, 75, or 150 mg or placebo for 14 days (in part 1 of the study) or VX-770 every 12 hours at a dose of 150 or 250 mg or placebo for 28 days (in part 2 of the study).
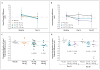
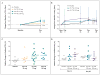
Results: At day 28, in the group of subjects who received 150 mg of VX-770, the median change in the nasal potential difference (in response to the administration of a chloride-free isoproterenol solution) from baseline was -3.5 mV (range, -8.3 to 0.5; P=0.02 for the within-subject comparison, P=0.13 vs. placebo), and the median change in the level of sweat chloride was -59.5 mmol per liter (range, -66.0 to -19.0; P=0.008 within-subject, P=0.02 vs. placebo). The median change from baseline in the percent of predicted forced expiratory volume in 1 second was 8.7% (range, 2.3 to 31.3; P=0.008 for the within-subject comparison, P=0.56 vs. placebo). None of the subjects withdrew from the study. Six severe adverse events occurred in two subjects (diffuse macular rash in one subject and five incidents of elevated blood and urine glucose levels in one subject with diabetes). All severe adverse events resolved without the discontinuation of VX-770.
Conclusions: This study to evaluate the safety and adverse-event profile of VX-770 showed that VX-770 was associated with within-subject improvements in CFTR and lung function. These findings provide support for further studies of pharmacologic potentiation of CFTR as a means to treat cystic fibrosis. (Funded by Vertex Pharmaceuticals and others; ClinicalTrials.gov number, NCT00457821.).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Targeting the basic defect in cystic fibrosis.N Engl J Med. 2010 Nov 18;363(21):2056-7. doi: 10.1056/NEJMe1010123. N Engl J Med. 2010. PMID: 21083391 No abstract available.
-
Cystic fibrosis transmembrane regulator potentiators as promising cystic fibrosis therapies.Expert Opin Investig Drugs. 2011 Mar;20(3):423-5. doi: 10.1517/13543784.2011.554823. Epub 2011 Feb 9. Expert Opin Investig Drugs. 2011. PMID: 21303308 Clinical Trial.
Similar articles
-
A CFTR potentiator in patients with cystic fibrosis and the G551D mutation.N Engl J Med. 2011 Nov 3;365(18):1663-72. doi: 10.1056/NEJMoa1105185. N Engl J Med. 2011. PMID: 22047557 Free PMC article. Clinical Trial.
-
VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles.N Engl J Med. 2018 Oct 25;379(17):1612-1620. doi: 10.1056/NEJMoa1807120. Epub 2018 Oct 18. N Engl J Med. 2018. PMID: 30334692 Free PMC article. Clinical Trial.
-
VX-659-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles.N Engl J Med. 2018 Oct 25;379(17):1599-1611. doi: 10.1056/NEJMoa1807119. Epub 2018 Oct 18. N Engl J Med. 2018. PMID: 30334693 Free PMC article. Clinical Trial.
-
Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2015 Mar 26;(3):CD009841. doi: 10.1002/14651858.CD009841.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jan 07;1:CD009841. doi: 10.1002/14651858.CD009841.pub3 PMID: 25811419 Updated. Review.
-
Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment.Ann Pharmacother. 2012 Jul-Aug;46(7-8):1065-75. doi: 10.1345/aph.1R076. Epub 2012 Jun 26. Ann Pharmacother. 2012. PMID: 22739718 Review.
Cited by
-
Chronic Pancreatitis in the 21st Century - Research Challenges and Opportunities: Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop.Pancreas. 2016 Nov;45(10):1365-1375. doi: 10.1097/MPA.0000000000000713. Pancreas. 2016. PMID: 27748719 Free PMC article.
-
CFTR: folding, misfolding and correcting the ΔF508 conformational defect.Trends Mol Med. 2012 Feb;18(2):81-91. doi: 10.1016/j.molmed.2011.10.003. Epub 2011 Dec 3. Trends Mol Med. 2012. PMID: 22138491 Free PMC article. Review.
-
Sources of Variation in Sweat Chloride Measurements in Cystic Fibrosis.Am J Respir Crit Care Med. 2016 Dec 1;194(11):1375-1382. doi: 10.1164/rccm.201603-0459OC. Am J Respir Crit Care Med. 2016. PMID: 27258095 Free PMC article.
-
Evaporimeter and Bubble-Imaging Measures of Sweat Gland Secretion Rates.PLoS One. 2016 Oct 21;11(10):e0165254. doi: 10.1371/journal.pone.0165254. eCollection 2016. PLoS One. 2016. PMID: 27768743 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis: current perspectives.Clin Pharmacol. 2016 Sep 21;8:127-140. doi: 10.2147/CPAA.S100759. eCollection 2016. Clin Pharmacol. 2016. PMID: 27703398 Free PMC article. Review.
References
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic & molecular bases of inherited disease. 8. Vol. 3. New York: McGraw-Hill; 2001. pp. 5121–88.
-
- Welsh MJ. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986;232:1648–50. - PubMed
-
- Patient registry: 2007 annual data report to the center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2008.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 RR025005/RR/NCRR NIH HHS/United States
- 1UL1 RR025744/RR/NCRR NIH HHS/United States
- UL1 RR025005-04/RR/NCRR NIH HHS/United States
- K23 DK075788-05/DK/NIDDK NIH HHS/United States
- UL1 RR025780-02/RR/NCRR NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- K23 DK075788/DK/NIDDK NIH HHS/United States
- UL 1RR025747/RR/NCRR NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- U01 HL081335-04/HL/NHLBI NIH HHS/United States
- UL1 RR025744-02/RR/NCRR NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- FD-R-003432-01/FD/FDA HHS/United States
- K23 RR018611/RR/NCRR NIH HHS/United States
- P30 DK072482/DK/NIDDK NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UL1 RR024134-05/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- P30 DK027651/DK/NIDDK NIH HHS/United States
- M01 RR002172-26/RR/NCRR NIH HHS/United States
- M01 RR002172/RR/NCRR NIH HHS/United States
- U01 HL081335/HL/NHLBI NIH HHS/United States
- UL1 RR025014-04/RR/NCRR NIH HHS/United States
- UL1 RR025747-02/RR/NCRR NIH HHS/United States
- M01 RR000070-41/RR/NCRR NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- M01 RR02172/RR/NCRR NIH HHS/United States
- M01 RR000070/RR/NCRR NIH HHS/United States
- UL1 RR024989-03/RR/NCRR NIH HHS/United States
- UL1 RR024153-05/RR/NCRR NIH HHS/United States
- K23 RR018611-05/RR/NCRR NIH HHS/United States
- M01 RR00070/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
