Novel sialic acid derivatives lock open the 150-loop of an influenza A virus group-1 sialidase
- PMID: 21081911
- PMCID: PMC3060605
- DOI: 10.1038/ncomms1114
Novel sialic acid derivatives lock open the 150-loop of an influenza A virus group-1 sialidase
Abstract
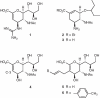
Influenza virus sialidase has an essential role in the virus' life cycle. Two distinct groups of influenza A virus sialidases have been established, that differ in the flexibility of the '150-loop', providing a more open active site in the apo form of the group-1 compared to group-2 enzymes. In this study we show, through a multidisciplinary approach, that novel sialic acid-based derivatives can exploit this structural difference and selectively inhibit the activity of group-1 sialidases. We also demonstrate that group-1 sialidases from drug-resistant mutant influenza viruses are sensitive to these designed compounds. Moreover, we have determined, by protein X-ray crystallography, that these inhibitors lock open the group-1 sialidase flexible 150-loop, in agreement with our molecular modelling prediction. This is the first direct proof that compounds may be developed to selectively target the pandemic A/H1N1, avian A/H5N1 and other group-1 sialidase-containing viruses, based on an open 150-loop conformation of the enzyme.
Figures


Similar articles
-
Synthesis and evaluation of novel 3-C-alkylated-Neu5Ac2en derivatives as probes of influenza virus sialidase 150-loop flexibility.Org Biomol Chem. 2012 Nov 21;10(43):8628-39. doi: 10.1039/c2ob25627d. Org Biomol Chem. 2012. PMID: 22976385
-
Novel 3,4-disubstituted-Neu5Ac2en derivatives as probes to investigate flexibility of the influenza virus sialidase 150-loop.Bioorg Med Chem. 2013 Aug 15;21(16):4820-30. doi: 10.1016/j.bmc.2013.05.054. Epub 2013 Jun 6. Bioorg Med Chem. 2013. PMID: 23800724
-
Complexity in influenza virus targeted drug design: interaction with human sialidases.J Med Chem. 2010 Apr 8;53(7):2998-3002. doi: 10.1021/jm100078r. J Med Chem. 2010. PMID: 20222714
-
Imaging of Sialidase Activity and Its Clinical Application.Biol Pharm Bull. 2017;40(12):2015-2023. doi: 10.1248/bpb.b17-00592. Biol Pharm Bull. 2017. PMID: 29199226 Review.
-
Comparative enzymology, biochemistry and pathophysiology of human exo-alpha-sialidases (neuraminidases).Comp Biochem Physiol B Biochem Mol Biol. 2001 May;129(1):29-64. doi: 10.1016/s1096-4959(01)00372-4. Comp Biochem Physiol B Biochem Mol Biol. 2001. PMID: 11337249 Review.
Cited by
-
Influenza Virus Neuraminidase Structure and Functions.Front Microbiol. 2019 Jan 29;10:39. doi: 10.3389/fmicb.2019.00039. eCollection 2019. Front Microbiol. 2019. PMID: 30761095 Free PMC article. Review.
-
Influenza A Virus Neuraminidase Inhibitors.Methods Mol Biol. 2022;2556:321-353. doi: 10.1007/978-1-0716-2635-1_21. Methods Mol Biol. 2022. PMID: 36175642
-
Targeting the host or the virus: current and novel concepts for antiviral approaches against influenza virus infection.Antiviral Res. 2012 Dec;96(3):391-404. doi: 10.1016/j.antiviral.2012.09.013. Epub 2012 Sep 26. Antiviral Res. 2012. PMID: 23022351 Free PMC article. Review.
-
Influenza A virus N5 neuraminidase has an extended 150-cavity.J Virol. 2011 Aug;85(16):8431-5. doi: 10.1128/JVI.00638-11. Epub 2011 Jun 8. J Virol. 2011. PMID: 21653672 Free PMC article.
-
Mesoscale All-Atom Influenza Virus Simulations Suggest New Substrate Binding Mechanism.ACS Cent Sci. 2020 Feb 26;6(2):189-196. doi: 10.1021/acscentsci.9b01071. Epub 2020 Feb 19. ACS Cent Sci. 2020. PMID: 32123736 Free PMC article.
References
-
- Zambon M. C. The pathogenesis of influenza in humans. Rev. Med. Virol. 11, 227–241 (2001). - PubMed
-
- World Health Organisation — Fact sheet No 211 (2009) Influenza. http://www.who.int/mediacentre/factsheets/fs211/en/.
-
- Wagner R., Matrosovich M. & Klenk H.- D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 12, 159–166 (2002). - PubMed
-
- Russell R. J. et al.. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 443, 45–49 (2006). - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

