Structure of Sir2Tm bound to a propionylated peptide
- PMID: 21080423
- PMCID: PMC3047069
- DOI: 10.1002/pro.544
Structure of Sir2Tm bound to a propionylated peptide
Abstract
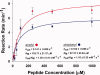
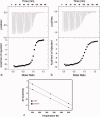
Lysine propionylation is a recently identified post-translational modification that has been observed in proteins such as p53 and histones and is thought to play a role similar to acetylation in modulating protein activity. Members of the sirtuin family of deacetylases have been shown to have depropionylation activity, although the way in which the sirtuin catalytic site accommodates the bulkier propionyl group is not clear. We have determined the 1.8 Å structure of a Thermotoga maritima sirtuin, Sir2Tm, bound to a propionylated peptide derived from p53. A comparison with the structure of Sir2Tm bound to an acetylated peptide shows that hydrophobic residues in the active site shift to accommodate the bulkier propionyl group. Isothermal titration calorimetry data show that Sir2Tm binds propionylated substrates more tightly than acetylated substrates, but kinetic assays reveal that the catalytic rate of Sir2Tm deacylation of propionyl-lysine is slightly reduced to acetyl-lysine. These results serve to broaden our understanding of the newly identified propionyl-lysine modification and the ability of sirtuins to depropionylate, as well as deacetylate, substrates.
Figures

Similar articles
-
Structure-based mechanism of ADP-ribosylation by sirtuins.J Biol Chem. 2009 Nov 27;284(48):33654-61. doi: 10.1074/jbc.M109.024521. Epub 2009 Sep 30. J Biol Chem. 2009. PMID: 19801667 Free PMC article.
-
N-lysine propionylation controls the activity of propionyl-CoA synthetase.J Biol Chem. 2007 Oct 12;282(41):30239-45. doi: 10.1074/jbc.M704409200. Epub 2007 Aug 7. J Biol Chem. 2007. PMID: 17684016
-
The structural basis of sirtuin substrate affinity.Biochemistry. 2006 Jun 20;45(24):7511-21. doi: 10.1021/bi0526332. Biochemistry. 2006. PMID: 16768447
-
A Molecular Perspective on Sirtuin Activity.Int J Mol Sci. 2020 Nov 15;21(22):8609. doi: 10.3390/ijms21228609. Int J Mol Sci. 2020. PMID: 33203121 Free PMC article. Review.
-
The ARF/oncogene pathway activates p53 acetylation within the DNA binding domain.Cell Cycle. 2007 Jun 1;6(11):1304-6. doi: 10.4161/cc.6.11.4343. Epub 2007 Jun 24. Cell Cycle. 2007. PMID: 17534149 Review.
Cited by
-
Protein N-terminal acetyltransferases act as N-terminal propionyltransferases in vitro and in vivo.Mol Cell Proteomics. 2013 Jan;12(1):42-54. doi: 10.1074/mcp.M112.019299. Epub 2012 Oct 4. Mol Cell Proteomics. 2013. PMID: 23043182 Free PMC article.
-
Biochemistry: Sirtuin on a high-fat diet.Nature. 2013 Apr 4;496(7443):41-2. doi: 10.1038/496041a. Nature. 2013. PMID: 23552941 No abstract available.
-
Sirtuin Deacetylation Mechanism and Catalytic Role of the Dynamic Cofactor Binding Loop.J Phys Chem Lett. 2013 Feb 7;4(3):491-495. doi: 10.1021/jz302015s. J Phys Chem Lett. 2013. PMID: 23585919 Free PMC article.
-
Molecular Mechanism of Sirtuin 1 Modulation by the AROS Protein.Int J Mol Sci. 2022 Oct 23;23(21):12764. doi: 10.3390/ijms232112764. Int J Mol Sci. 2022. PMID: 36361557 Free PMC article.
-
Propionate induces intestinal oxidative stress via Sod2 propionylation in zebrafish.iScience. 2021 May 5;24(6):102515. doi: 10.1016/j.isci.2021.102515. eCollection 2021 Jun 25. iScience. 2021. PMID: 34142031 Free PMC article.
References
-
- Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. - PubMed
-
- Garrity J, Gardner JG, Hawse W, Wolberger C, Escalante-Semerena JC. N-lysine propionylation controls the activity of propionyl-CoA synthetase. J Biol Chem. 2007;282:30239–30245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

