The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5
- PMID: 21076070
- PMCID: PMC3058366
- DOI: 10.4049/jimmunol.1002803
The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5
Abstract
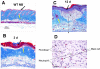
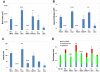
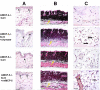
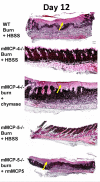
A second-degree epidermal scald burn in mice elicits an inflammatory response mediated by natural IgM directed to nonmuscle myosin with complement activation that results in ulceration and scarring. We find that such burn injury is associated with early mast cell (MC) degranulation and is absent in WBB6F1-Kit(W)/Kit(Wv) mice, which lack MCs in a context of other defects due to a mutation of the Kit receptor. To address further an MC role, we used transgenic strains with normal lineage development and a deficiency in a specific secretory granule component. Mouse strains lacking the MC-restricted chymase, mouse MC protease (mMCP)-4, or elastase, mMCP-5, show decreased injury after a second-degree scald burn, whereas mice lacking the MC-restricted tryptases, mMCP-6 and mMCP-7, or MC-specific carboxypeptidase A3 activity are not protected. Histologic sections showed some disruption of the epidermis at the scald site in the protected strains suggesting the possibility of topical reconstitution of full injury. Topical application of recombinant mMCP-5 or human neutrophil elastase to the scalded area increases epidermal injury with subsequent ulceration and scarring, both clinically and morphologically, in mMCP-5-deficient mice. Restoration of injury requires that topical administration of recombinant mMCP-5 occurs within the first hour postburn. Importantly, topical application of human MC chymase restores burn injury to scalded mMCP-4-deficient mice but not to mMCP-5-deficient mice revealing nonredundant actions for these two MC proteases in a model of innate inflammatory injury with remodeling.
Figures




Similar articles
-
Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle.J Immunol. 2005 Jun 1;174(11):7285-91. doi: 10.4049/jimmunol.174.11.7285. J Immunol. 2005. PMID: 15905575 Free PMC article.
-
Mouse bone marrow-derived mast cells (mBMMC) obtained in vitro from mice that are mast cell-deficient in vivo express the same panel of granule proteases as mBMMC and serosal mast cells from their normal littermates.J Exp Med. 1994 Jul 1;180(1):67-73. doi: 10.1084/jem.180.1.67. J Exp Med. 1994. PMID: 8006601 Free PMC article.
-
Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions.J Immunol. 2014 Mar 15;192(6):2812-20. doi: 10.4049/jimmunol.1301794. Epub 2014 Feb 12. J Immunol. 2014. PMID: 24523504 Free PMC article.
-
The emerging role of mast cell proteases in asthma.Eur Respir J. 2019 Oct 31;54(4):1900685. doi: 10.1183/13993003.00685-2019. Print 2019 Oct. Eur Respir J. 2019. PMID: 31371445 Review.
-
Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity.Immunol Rev. 2007 Jun;217:155-67. doi: 10.1111/j.1600-065X.2007.00525.x. Immunol Rev. 2007. PMID: 17498058 Review.
Cited by
-
Skin Telocytes Could Fundament the Cellular Mechanisms of Wound Healing in Platelet-Rich Plasma Administration.Cells. 2024 Aug 8;13(16):1321. doi: 10.3390/cells13161321. Cells. 2024. PMID: 39195210 Free PMC article. Review.
-
The multifaceted mast cell in inflammatory bowel disease.Inflamm Bowel Dis. 2014 Dec;20(12):2364-78. doi: 10.1097/MIB.0000000000000142. Inflamm Bowel Dis. 2014. PMID: 25401721 Free PMC article. Review.
-
A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis.Int J Mol Sci. 2020 Dec 18;21(24):9673. doi: 10.3390/ijms21249673. Int J Mol Sci. 2020. PMID: 33353063 Free PMC article. Review.
-
Approaches for analyzing the roles of mast cells and their proteases in vivo.Adv Immunol. 2015;126:45-127. doi: 10.1016/bs.ai.2014.11.002. Epub 2015 Feb 7. Adv Immunol. 2015. PMID: 25727288 Free PMC article. Review.
-
Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice.PLoS One. 2013;8(3):e59167. doi: 10.1371/journal.pone.0059167. Epub 2013 Mar 27. PLoS One. 2013. PMID: 23544053 Free PMC article.
References
-
- Horton JW, Mileski WJ, White DJ, Lipsky P. Monoclonal Antibody to Intercellular Adhesion Molecule-1 Reduces Cardiac Contractile Dysfunction after Burn Injury in Rabbits. J. Surg. Res. 1996;64:49–56. - PubMed
-
- Nwariaku FEM, Mileski WJM, Lightfoot EJ, Sikes PJB, Lipsky PEM. Alterations in Leukocyte Adhesion Molecule Expression after Burn Injury. J. Trauma. 1995;39:285–288. - PubMed
-
- Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, Carroll MC, Moore FD, Jr., Austen WG., Jr. The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J. Immunol. 2006;177:8080–8085. - PubMed
-
- Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD., Jr. Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139:236–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

