MCP-1 gene activation marks acute kidney injury
- PMID: 21071523
- PMCID: PMC3014045
- DOI: 10.1681/ASN.2010060641
MCP-1 gene activation marks acute kidney injury
Abstract
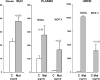
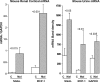
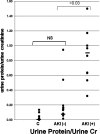
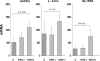
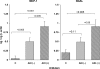
Monocyte chemoattractant protein 1 (MCP-1) mediates acute ischemic and toxic kidney injury, but whether this can be used as a biomarker of acute kidney injury (AKI) is unknown. We obtained kidney and urine samples from mice with intrarenal (maleate), prerenal (endotoxemia), or postrenal (ureteral obstruction) injury. We also studied the independent effects of uremia without concomitant kidney injury by performing bilateral ureteral transection in mice. Additionally, we obtained urine samples from APACHE II-matched critically ill patients with or without advancing azotemia (n = 10 in each group). We assayed selected samples for MCP-1, MCP-1 mRNA, and for an activating histone mark (H3K4m3) at urinary fragments of the MCP-1 gene and contrasted the results with those obtained for neutrophil gelatinase-associated lipocalin (NGAL), a comparator "AKI biomarker" gene. Maleate increased urinary MCP-1 protein and mRNA more than the corresponding increases in NGAL. Endotoxemia and ureteral obstruction also increased NGAL and MCP-1 gene expression. Uremia, in the absence of renal injury, induced the NGAL gene, but not MCP-1, suggesting the possibility of better specificity of MCP-1 for AKI. Clinical assessments supported the utility of MCP-1 as a biomarker (e.g., nonoverlapping concentrations of urinary MCP-1 in patients with and without AKI). Elevated levels of urinary MCP-1 mRNA and levels of H3K4m3 at the MCP-1 gene supported MCP-1 gene activation in patients with renal injury. In conclusion, these data suggest that MCP-1 has potential as a biomarker of AKI and provide "proof of concept" that urinary histone assessments provide mechanistic insight among patients with kidney disease.
Figures





Similar articles
-
Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury.Am J Physiol Renal Physiol. 2010 Aug;299(2):F426-35. doi: 10.1152/ajprenal.00248.2010. Epub 2010 May 26. Am J Physiol Renal Physiol. 2010. PMID: 20504881 Free PMC article.
-
Serum and urinary NGAL but not KIM-1 raises in human postrenal AKI.Eur J Clin Invest. 2014 Jul;44(7):652-9. doi: 10.1111/eci.12283. Eur J Clin Invest. 2014. PMID: 24837251
-
Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons.Kidney Int. 2009 Feb;75(3):285-94. doi: 10.1038/ki.2008.499. Epub 2008 Oct 1. Kidney Int. 2009. PMID: 19148153
-
Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis.Am J Kidney Dis. 2009 Dec;54(6):1012-24. doi: 10.1053/j.ajkd.2009.07.020. Epub 2009 Oct 21. Am J Kidney Dis. 2009. PMID: 19850388 Review.
-
Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences.Clin Chem Lab Med. 2012 Feb 15;50(9):1505-17. doi: 10.1515/cclm-2011-0814. Clin Chem Lab Med. 2012. PMID: 22962216 Review.
Cited by
-
Management of Acute Kidney Injury Following Major Abdominal Surgery: A Contemporary Review.J Clin Med. 2020 Aug 18;9(8):2679. doi: 10.3390/jcm9082679. J Clin Med. 2020. PMID: 32824854 Free PMC article. Review.
-
Human AKI and heme oxygenase-1.J Am Soc Nephrol. 2012 Jun;23(6):971-4. doi: 10.1681/ASN.2012040380. Epub 2012 May 10. J Am Soc Nephrol. 2012. PMID: 22581993 Free PMC article. No abstract available.
-
Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement.J Am Soc Nephrol. 2012 Jul;23(7):1161-71. doi: 10.1681/ASN.2011050442. Epub 2012 Apr 19. J Am Soc Nephrol. 2012. PMID: 22518005 Free PMC article.
-
Current developments in early diagnosis of acute kidney injury.Int Urol Nephrol. 2014 Jan;46(1):1-7. doi: 10.1007/s11255-013-0448-5. Epub 2013 May 15. Int Urol Nephrol. 2014. PMID: 23673775 Review.
-
HMG-CoA reductase activation and urinary pellet cholesterol elevations in acute kidney injury.Clin J Am Soc Nephrol. 2011 Sep;6(9):2108-13. doi: 10.2215/CJN.02440311. Epub 2011 Jul 28. Clin J Am Soc Nephrol. 2011. PMID: 21799150 Free PMC article.
References
-
- Zager RA, Carpenter CB: Radioimmunoassay for urinary renal tubular antigen: A potential marker of tubular injury. Kidney Int 13: 505–512, 1978 - PubMed
-
- Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob HG, Philipp T, Kribben A: Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem 50: 552–558, 2004 - PubMed
-
- Blaikley J, Sutton P, Walter M, Lapsley M, Norden A, Pugsley W, Unwin R: Tubular proteinuria and enzymuria following open heart surgery. Intensive Care Med 29: 1364–1367, 2003 - PubMed
-
- Kharasch ED, Frink EJ, Jr., Zager R, Bowdle TA, Artru A, Nogami WM: Assessment of low-flow sevoflurane and isoflurane effects on renal function using sensitive markers of tubular toxicity. Anesthesiology 86: 1238–1253, 1997 - PubMed
-
- Zager RA: Urinary protein markers of tubulointerstitial nephritis. Invest Urol 8: 197–202, 1980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

