Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin
- PMID: 21071447
- PMCID: PMC3020718
- DOI: 10.1074/jbc.M110.178806
Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin
Abstract
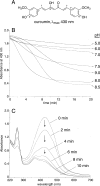
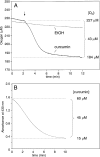
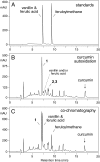
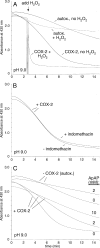
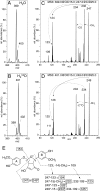
The efficacy of the diphenol curcumin as a cancer chemopreventive agent is limited by its chemical and metabolic instability. Non-enzymatic degradation has been described to yield vanillin, ferulic acid, and feruloylmethane through cleavage of the heptadienone chain connecting the phenolic rings. Here we provide evidence for an alternative mechanism, resulting in autoxidative cyclization of the heptadienone moiety as a major pathway of degradation. Autoxidative transformation of curcumin was pH-dependent with the highest rate at pH 8 (2.2 μM/min) and associated with stoichiometric uptake of O(2). Oxidation was also catalyzed by recombinant cyclooxygenase-2 (COX-2) (50 nm; 7.5 μM/min), and the rate was increased ≈10-fold by the addition of 300 μM H(2)O(2). The COX-2 catalyzed transformation was inhibited by acetaminophen but not indomethacin, suggesting catalysis occurred by the peroxidase activity. We propose a mechanism of enzymatic or autoxidative hydrogen abstraction from a phenolic hydroxyl to give a quinone methide and a delocalized radical in the heptadienone chain that undergoes 5-exo cyclization and oxygenation. Hydration of the quinone methide (measured by the incorporation of O-18 from H(2)(18)O) and rearrangement under loss of water gives the final dioxygenated bicyclopentadione product. When curcumin was added to RAW264.7 cells, the bicyclopentadione was increased 1.8-fold in cells activated by LPS; vanillin and other putative cleavage products were negligible. Oxidation to a reactive quinone methide is the mechanistic basis of many phenolic anti-cancer drugs. It is possible, therefore, that oxidative transformation of curcumin, a prominent but previously unrecognized reaction, contributes to its cancer chemopreventive activity.
Figures



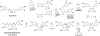
Similar articles
-
Oxidative metabolites of curcumin poison human type II topoisomerases.Biochemistry. 2013 Jan 8;52(1):221-7. doi: 10.1021/bi3014455. Epub 2012 Dec 26. Biochemistry. 2013. PMID: 23253398 Free PMC article.
-
Oxidative metabolism of curcumin-glucuronide by peroxidases and isolated human leukocytes.Biochem Pharmacol. 2017 May 15;132:143-149. doi: 10.1016/j.bcp.2017.03.002. Epub 2017 Mar 6. Biochem Pharmacol. 2017. PMID: 28274615 Free PMC article.
-
Unraveling curcumin degradation: autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione.J Biol Chem. 2015 Feb 20;290(8):4817-4828. doi: 10.1074/jbc.M114.618785. Epub 2015 Jan 6. J Biol Chem. 2015. PMID: 25564617 Free PMC article.
-
Degradation of Curcumin: From Mechanism to Biological Implications.J Agric Food Chem. 2015 Sep 9;63(35):7606-14. doi: 10.1021/acs.jafc.5b00244. Epub 2015 Apr 2. J Agric Food Chem. 2015. PMID: 25817068 Free PMC article. Review.
-
Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation.Mutat Res. 2001 Sep 1;480-481:243-68. doi: 10.1016/s0027-5107(01)00183-x. Mutat Res. 2001. PMID: 11506818 Review.
Cited by
-
Dry Heating of Curcumin in the Presence of Basic Salts Yields Anti-inflammatory Dimerization Products.ACS Omega. 2024 Aug 20;9(35):37025-37034. doi: 10.1021/acsomega.4c03257. eCollection 2024 Sep 3. ACS Omega. 2024. PMID: 39246485 Free PMC article.
-
Dietary Effect of Curcumin on Amino Acid, Fatty Acid, and Volatile Compound Profiles of Chicken Meat.Foods. 2024 Jul 16;13(14):2230. doi: 10.3390/foods13142230. Foods. 2024. PMID: 39063312 Free PMC article.
-
A Realistic View on "The Essential Medicinal Chemistry of Curcumin".ACS Med Chem Lett. 2017 Sep 14;8(9):893-896. doi: 10.1021/acsmedchemlett.7b00284. eCollection 2017 Sep 14. ACS Med Chem Lett. 2017. PMID: 28947929 Free PMC article. No abstract available.
-
Dietary Curcumin Improves Energy Metabolism, Brain Monoamines, Carcass Traits, Muscle Oxidative Stability and Fatty Acid Profile in Heat-Stressed Broiler Chickens.Antioxidants (Basel). 2021 Aug 9;10(8):1265. doi: 10.3390/antiox10081265. Antioxidants (Basel). 2021. PMID: 34439513 Free PMC article.
-
Curcumin suppresses colorectal tumorigenesis via the Wnt/β-catenin signaling pathway by downregulating Axin2.Oncol Lett. 2021 Mar;21(3):186. doi: 10.3892/ol.2021.12447. Epub 2021 Jan 6. Oncol Lett. 2021. PMID: 33574925 Free PMC article.
References
-
- Aggarwal B. B., Sung B. (2009) Trends Pharmacol. Sci. 30, 85–94 - PubMed
-
- Aggarwal B. B., Shishodia S. (2006) Biochem. Pharmacol. 71, 1397–1421 - PubMed
-
- Tønnesen H. H., Karlsen J., van Henegouwen G. B. (1986) Z. Lebensm. Unters. Forsch. 183, 116–122 - PubMed
-
- Khurana A., Ho C. T. (1988) J. Liquid Chromatogr. 11, 2295–2304
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

