Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration
- PMID: 21068842
- PMCID: PMC3058628
- DOI: 10.1038/nature09493
Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration
Abstract
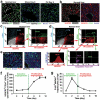
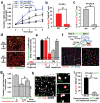
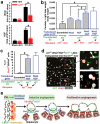
During embryogenesis, endothelial cells induce organogenesis before the development of circulation. These findings suggest that endothelial cells not only form passive conduits to deliver nutrients and oxygen, but also establish an instructive vascular niche, which through elaboration of paracrine trophogens stimulates organ regeneration, in a manner similar to endothelial-cell-derived angiocrine factors that support haematopoiesis. However, the precise mechanism by which tissue-specific subsets of endothelial cells promote organogenesis in adults is unknown. Here we demonstrate that liver sinusoidal endothelial cells (LSECs) constitute a unique population of phenotypically and functionally defined VEGFR3(+)CD34(-)VEGFR2(+)VE-cadherin(+)FactorVIII(+)CD45(-) endothelial cells, which through the release of angiocrine trophogens initiate and sustain liver regeneration induced by 70% partial hepatectomy. After partial hepatectomy, residual liver vasculature remains intact without experiencing hypoxia or structural damage, which allows study of physiological liver regeneration. Using this model, we show that inducible genetic ablation of vascular endothelial growth factor (VEGF)-A receptor-2 (VEGFR2) in the LSECs impairs the initial burst of hepatocyte proliferation (days 1-3 after partial hepatectomy) and subsequent reconstitution of the hepatovascular mass (days 4-8 after partial hepatectomy) by inhibiting upregulation of the endothelial-cell-specific transcription factor Id1. Accordingly, Id1-deficient mice also manifest defects throughout liver regeneration, owing to diminished expression of LSEC-derived angiocrine factors, including hepatocyte growth factor (HGF) and Wnt2. Notably, in in vitro co-cultures, VEGFR2-Id1 activation in LSECs stimulates hepatocyte proliferation. Indeed, intrasplenic transplantation of Id1(+/+) or Id1(-/-) LSECs transduced with Wnt2 and HGF (Id1(-/-)Wnt2(+)HGF(+) LSECs) re-establishes an inductive vascular niche in the liver sinusoids of the Id1(-/-) mice, initiating and restoring hepatovascular regeneration. Therefore, in the early phases of physiological liver regeneration, VEGFR2-Id1-mediated inductive angiogenesis in LSECs through release of angiocrine factors Wnt2 and HGF provokes hepatic proliferation. Subsequently, VEGFR2-Id1-dependent proliferative angiogenesis reconstitutes liver mass. Therapeutic co-transplantation of inductive VEGFR2(+)Id1(+)Wnt2(+)HGF(+) LSECs with hepatocytes provides an effective strategy to achieve durable liver regeneration.
Figures

Comment in
-
Key role of sinusoidal endothelial cells in the triggering of liver regeneration.J Hepatol. 2011 Aug;55(2):488-90. doi: 10.1016/j.jhep.2011.02.005. Epub 2011 Feb 22. J Hepatol. 2011. PMID: 21349303 No abstract available.
-
Sinusoidal endothelium is essential for liver regeneration.Hepatology. 2011 Aug;54(2):731-3. doi: 10.1002/hep.24455. Hepatology. 2011. PMID: 21793020 No abstract available.
Similar articles
-
The effect of aging on VEGF/VEGFR2 signal pathway genes expression in rat liver sinusoidal endothelial cell.Mol Cell Biochem. 2021 Jan;476(1):269-277. doi: 10.1007/s11010-020-03903-7. Epub 2020 Sep 12. Mol Cell Biochem. 2021. PMID: 32918705
-
Angiocrine Hepatocyte Growth Factor Signaling Controls Physiological Organ and Body Size and Dynamic Hepatocyte Proliferation to Prevent Liver Damage during Regeneration.Am J Pathol. 2020 Feb;190(2):358-371. doi: 10.1016/j.ajpath.2019.10.009. Epub 2019 Nov 27. Am J Pathol. 2020. PMID: 31783007
-
Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis.Nature. 2014 Jan 2;505(7481):97-102. doi: 10.1038/nature12681. Epub 2013 Nov 20. Nature. 2014. PMID: 24256728 Free PMC article.
-
The vascular endothelial growth factor signaling pathway regulates liver sinusoidal endothelial cells during liver regeneration after partial hepatectomy.Expert Rev Gastroenterol Hepatol. 2021 Feb;15(2):139-147. doi: 10.1080/17474124.2020.1815532. Epub 2020 Oct 14. Expert Rev Gastroenterol Hepatol. 2021. PMID: 32902336 Review.
-
Liver sinusoidal endothelial cells and liver regeneration.J Clin Invest. 2013 May;123(5):1861-6. doi: 10.1172/JCI66025. Epub 2013 May 1. J Clin Invest. 2013. PMID: 23635783 Free PMC article. Review.
Cited by
-
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks.J Gastrointest Surg. 2013 Apr;17(4):814-21. doi: 10.1007/s11605-012-2092-2. Epub 2012 Nov 27. J Gastrointest Surg. 2013. PMID: 23188224
-
Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice.Hepatology. 2012 May;55(5):1562-73. doi: 10.1002/hep.24800. Epub 2012 Mar 18. Hepatology. 2012. PMID: 22105716 Free PMC article.
-
Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments.J Hepatol. 2015 Apr;62(1 Suppl):S121-30. doi: 10.1016/j.jhep.2015.01.003. J Hepatol. 2015. PMID: 25920081 Free PMC article. Review.
-
Fibronectin Extra Domain A Promotes Liver Sinusoid Repair following Hepatectomy.PLoS One. 2016 Oct 14;11(10):e0163737. doi: 10.1371/journal.pone.0163737. eCollection 2016. PLoS One. 2016. PMID: 27741254 Free PMC article.
-
Transcriptomic Characterization of Key Factors and Signaling Pathways for the Regeneration of Partially Hepatectomized Liver in Zebrafish.Int J Mol Sci. 2024 Jun 29;25(13):7212. doi: 10.3390/ijms25137212. Int J Mol Sci. 2024. PMID: 39000319 Free PMC article.
References
-
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. - PubMed
-
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. - PubMed
-
- Butler JN, Vertes J, Varnum-Finney E, Kobayashi B, Hooper H, Seandel A, Shido M, White K, Kobayashi I, Witte M, May L, Shawber C, Kimura C, Kitajewski Y, Rosenwaks J, Bernstein Z, Rafii I, Endothelial S. Cells Are Essential for the Self-Renewal and Repopulation of Notch-Dependent Hematopoietic Stem Cells. Cell Stem Cell. 2010;6:1–14. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- P01 HL059312-090006/HL/NHLBI NIH HHS/United States
- R01 HL097797-03/HL/NHLBI NIH HHS/United States
- P50 HL084936/HL/NHLBI NIH HHS/United States
- P01 HL059312-100006/HL/NHLBI NIH HHS/United States
- P50 HL084936-010003/HL/NHLBI NIH HHS/United States
- R01 HL097797-01/HL/NHLBI NIH HHS/United States
- RC1 AI080309/AI/NIAID NIH HHS/United States
- P01 HL059312/HL/NHLBI NIH HHS/United States
- U01 HL-66592-03/HL/NHLBI NIH HHS/United States
- P50 HL084936-040003/HL/NHLBI NIH HHS/United States
- HHMI_/Howard Hughes Medical Institute/United States
- P01 HL067839/HL/NHLBI NIH HHS/United States
- P50 HL084936-030003/HL/NHLBI NIH HHS/United States
- R01 HL097797-02/HL/NHLBI NIH HHS/United States
- R01 HL097797/HL/NHLBI NIH HHS/United States
- P50 HL084936-020003/HL/NHLBI NIH HHS/United States
- HL097797/HL/NHLBI NIH HHS/United States
- P01 HL067839-050004/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

