The retroviral cyclin of walleye dermal sarcoma virus binds cyclin-dependent kinases 3 and 8
- PMID: 21067790
- PMCID: PMC3008307
- DOI: 10.1016/j.virol.2010.10.022
The retroviral cyclin of walleye dermal sarcoma virus binds cyclin-dependent kinases 3 and 8
Abstract
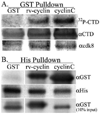
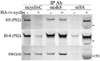
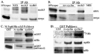
Walleye dermal sarcoma virus encodes a retroviral cyclin (rv-cyclin) with a cyclin box fold and transcription activation domain (AD). Co-immune precipitation (co-IP) identified an association of rv-cyclin with cyclin-dependent kinase 8 (cdk8). Cdk8 is dependent upon cyclin C and regulates transcription with the Mediator complex, a co-activator of transcription. Mutation of cyclin residues, required for cdk binding, disrupts rv-cyclin-cdk8 co-IP. Mutation or removal of the AD has no effect on cdk8 interaction. Direct rv-cyclin-cdk8 binding is demonstrated by pulldown of active cdk8 and by GST-rv-cyclin binding to recombinant cdk8. Cdk3 is also activated by cyclin C and phosphorylates retinoblastoma protein to initiate entry into the cell division cycle. Co-IP and pulldowns demonstrate direct rv-cyclin binding to cdk3 as well. The rv-cyclin functions as a structural ortholog of cyclin C in spite of its limited amino acid sequence identity with C cyclins or with any known cyclins.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Retroviral cyclin controls cyclin-dependent kinase 8-mediated transcription elongation and reinitiation.J Virol. 2015 May;89(10):5450-61. doi: 10.1128/JVI.00464-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25741012 Free PMC article.
-
Retroviral cyclin enhances cyclin-dependent kinase-8 activity.J Virol. 2012 May;86(10):5742-51. doi: 10.1128/JVI.07006-11. Epub 2012 Feb 29. J Virol. 2012. PMID: 22379099 Free PMC article.
-
Walleye dermal sarcoma virus retroviral cyclin directly contacts TAF9.J Virol. 2006 Dec;80(24):12041-8. doi: 10.1128/JVI.01425-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035330 Free PMC article.
-
CDK8: a positive regulator of transcription.Transcription. 2010 Jul-Aug;1(1):4-12. doi: 10.4161/trns.1.1.12373. Transcription. 2010. PMID: 21327159 Free PMC article. Review.
-
The cyclin C/Cdk8 kinase.Prog Cell Cycle Res. 1996;2:197-204. doi: 10.1007/978-1-4615-5873-6_19. Prog Cell Cycle Res. 1996. PMID: 9552396 Review.
Cited by
-
Cyclin-Dependent Kinases 8 and 19 Regulate Host Cell Metabolism during Dengue Virus Serotype 2 Infection.Viruses. 2020 Jun 17;12(6):654. doi: 10.3390/v12060654. Viruses. 2020. PMID: 32560467 Free PMC article.
-
Breaking Bad: How Viruses Subvert the Cell Cycle.Front Cell Infect Microbiol. 2018 Nov 19;8:396. doi: 10.3389/fcimb.2018.00396. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30510918 Free PMC article. Review.
-
Dysregulation of CDK8 and Cyclin C in tumorigenesis.J Genet Genomics. 2011 Oct 20;38(10):439-52. doi: 10.1016/j.jgg.2011.09.002. Epub 2011 Sep 16. J Genet Genomics. 2011. PMID: 22035865 Free PMC article. Review.
-
Exploitation of the Mediator complex by viruses.PLoS Pathog. 2022 Apr 21;18(4):e1010422. doi: 10.1371/journal.ppat.1010422. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35446926 Free PMC article. No abstract available.
-
Walleye dermal sarcoma virus: expression of a full-length clone or the rv-cyclin (orf a) gene is cytopathic to the host and human tumor cells.Mol Biol Rep. 2013 Feb;40(2):1451-61. doi: 10.1007/s11033-012-2188-5. Epub 2012 Oct 26. Mol Biol Rep. 2013. PMID: 23100064
References
-
- Bowser PR, Wooster GA, Quackenbush SL, Casey RN, Casey JW. Comparison of fall and spring tumors as inocula for experimental transmission of walleye dermal sarcoma. J. Aquat. Anim. Health. 1996;8:78–81.
-
- Ceruti JM, Scassa ME, Flo JM, Varone CL, Canepa ET. Induction of p19INK4d in response to ultraviolet light improves DNA repair and confers resistance to apoptosis in neuroblastoma cells. Oncogene. 2005;24:4065–4080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

