The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus
- PMID: 21056098
- PMCID: PMC3078527
- DOI: 10.1016/j.heares.2010.10.018
The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus
Abstract
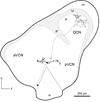
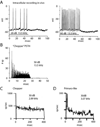
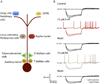
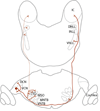
Acoustic information is brought to the brain by auditory nerve fibers, all of which terminate in the cochlear nuclei, and is passed up the auditory pathway through the principal cells of the cochlear nuclei. A population of neurons variously known as T stellate, type I multipolar, planar multipolar, or chopper cells forms one of the major ascending auditory pathways through the brainstem. T Stellate cells are sharply tuned; as a population they encode the spectrum of sounds. In these neurons, phasic excitation from the auditory nerve is made more tonic by feedforward excitation, coactivation of inhibitory with excitatory inputs, relatively large excitatory currents through NMDA receptors, and relatively little synaptic depression. The mechanisms that make firing tonic also obscure the fine structure of sounds that is represented in the excitatory inputs from the auditory nerve and account for the characteristic chopping response patterns with which T stellate cells respond to tones. In contrast with other principal cells of the ventral cochlear nucleus (VCN), T stellate cells lack a low-voltage-activated potassium conductance and are therefore sensitive to small, steady, neuromodulating currents. The presence of cholinergic, serotonergic and noradrenergic receptors allows the excitability of these cells to be modulated by medial olivocochlear efferent neurons and by neuronal circuits associated with arousal. T Stellate cells deliver acoustic information to the ipsilateral dorsal cochlear nucleus (DCN), ventral nucleus of the trapezoid body (VNTB), periolivary regions around the lateral superior olivary nucleus (LSO), and to the contralateral ventral lemniscal nuclei (VNLL) and inferior colliculus (IC). It is likely that T stellate cells participate in feedback loops through both medial and lateral olivocochlear efferent neurons and they may be a source of ipsilateral excitation of the LSO.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Synaptic inputs to stellate cells in the ventral cochlear nucleus.J Neurophysiol. 1998 Jan;79(1):51-63. doi: 10.1152/jn.1998.79.1.51. J Neurophysiol. 1998. PMID: 9425176
-
Synaptic Inhibition of Medial Olivocochlear Efferent Neurons by Neurons of the Medial Nucleus of the Trapezoid Body.J Neurosci. 2020 Jan 15;40(3):509-525. doi: 10.1523/JNEUROSCI.1288-19.2019. Epub 2019 Nov 12. J Neurosci. 2020. PMID: 31719165 Free PMC article.
-
Projections of the trapezoid body and the superior olivary complex of the Kangaroo rat (Dipodomys merriami).Brain Behav Evol. 1975;11(5-6):322-54. doi: 10.1159/000123643. Brain Behav Evol. 1975. PMID: 1192176
-
Afferent projections of the superior olivary complex.Microsc Res Tech. 2000 Nov 15;51(4):330-54. doi: 10.1002/1097-0029(20001115)51:4<330::AID-JEMT4>3.0.CO;2-X. Microsc Res Tech. 2000. PMID: 11071718 Review.
-
Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei.Brain Res Bull. 2003 Jun 15;60(5-6):457-74. doi: 10.1016/s0361-9230(03)00050-9. Brain Res Bull. 2003. PMID: 12787867 Review.
Cited by
-
Optogenetically modified human embryonic stem cell-derived otic neurons establish functional synaptic connection with cochlear nuclei.J Tissue Eng. 2024 Jul 31;15:20417314241265198. doi: 10.1177/20417314241265198. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 39092452 Free PMC article.
-
ERG Channels Regulate Excitability in Stellate and Bushy Cells of Mice Ventral Cochlear Nucleus.J Membr Biol. 2018 Dec;251(5-6):711-722. doi: 10.1007/s00232-018-0048-5. Epub 2018 Sep 11. J Membr Biol. 2018. PMID: 30206647
-
Kv1 channels regulate variations in spike patterning and temporal reliability in the avian cochlear nucleus angularis.J Neurophysiol. 2022 Jan 1;127(1):116-129. doi: 10.1152/jn.00460.2021. Epub 2021 Nov 24. J Neurophysiol. 2022. PMID: 34817286 Free PMC article.
-
Immediate manifestation of acoustic trauma in the auditory cortex is layer specific and cell type dependent.J Neurophysiol. 2016 Apr;115(4):1860-74. doi: 10.1152/jn.00810.2015. Epub 2016 Jan 28. J Neurophysiol. 2016. PMID: 26823513 Free PMC article.
-
Onset- and offset-specific effects in interaural level difference discrimination.J Acoust Soc Am. 2012 Sep;132(3):1573-80. doi: 10.1121/1.4740496. J Acoust Soc Am. 2012. PMID: 22978886 Free PMC article.
References
-
- Adams JC. Ascending projections to the inferior colliculus. J. Comp. Neurol. 1979;183:519–538. - PubMed
-
- Adams JC, Mugnaini E. Patterns of glutamate decarboxylase immunostaining in the feline cochlear nuclear complex studied with silver enhancement and electron microscopy. Journal.of.Comparative.Neurology. 1987;262:375–401. - PubMed
-
- Adams JC, Warr WB. Origins of axons in the cat's acoustic striae determined by injection of horseradish peroxidase into severed tracts. J. Comp. Neurol. 1976;170:107–121. - PubMed
-
- Alibardi L. Ultrastructural and immunocytochemical characterization of neurons in the rat ventral cochlear nucleus projecting to the inferior colliculus. Ann. Anat. 1998;180:415–426. - PubMed
-
- Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nuclei. J. Neurophysiol. 2001;86:2299–2311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

