The ubiquitous role of ubiquitin in the DNA damage response
- PMID: 21056014
- PMCID: PMC7105183
- DOI: 10.1016/j.dnarep.2010.09.011
The ubiquitous role of ubiquitin in the DNA damage response
Abstract
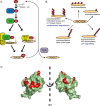
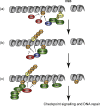
Protein ubiquitylation has emerged as an important regulatory mechanism that impacts almost every aspect of the DNA damage response. In this review, we discuss how DNA repair and checkpoint pathways utilize the diversity offered by the ubiquitin conjugation system to modulate the response to genotoxic lesions in space and time. In particular, we will highlight recent work done on the regulation of DNA double-strand breaks signalling and repair by the RNF8/RNF168 E3 ubiquitin ligases, the Fanconi anemia pathway and the role of protein degradation in the enforcement and termination of checkpoint signalling. We also discuss the various functions of deubiquitylating enzymes in these processes along with potential avenues for exploiting the ubiquitin conjugation/deconjugation system for therapeutic purposes.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
The p97-Ataxin 3 complex regulates homeostasis of the DNA damage response E3 ubiquitin ligase RNF8.EMBO J. 2019 Oct 4;38(21):e102361. doi: 10.15252/embj.2019102361. Epub 2019 Oct 15. EMBO J. 2019. PMID: 31613024 Free PMC article.
-
Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage.Nature. 2015 Nov 19;527(7578):389-93. doi: 10.1038/nature15401. Epub 2015 Oct 21. Nature. 2015. PMID: 26503038
-
HP1 regulates the localization of FANCJ at sites of DNA double-strand breaks.Cancer Sci. 2016 Oct;107(10):1406-1415. doi: 10.1111/cas.13008. Epub 2016 Sep 1. Cancer Sci. 2016. PMID: 27399284 Free PMC article.
-
The ubiquitin- and SUMO-dependent signaling response to DNA double-strand breaks.FEBS Lett. 2011 Sep 16;585(18):2914-9. doi: 10.1016/j.febslet.2011.05.056. Epub 2011 Jun 12. FEBS Lett. 2011. PMID: 21664912 Review.
-
Regulatory ubiquitylation in response to DNA double-strand breaks.DNA Repair (Amst). 2009 Apr 5;8(4):436-43. doi: 10.1016/j.dnarep.2009.01.013. Epub 2009 Feb 18. DNA Repair (Amst). 2009. PMID: 19230794 Review.
Cited by
-
53BP1 is limiting for NHEJ repair in ATM-deficient model systems that are subjected to oncogenic stress or radiation.Mol Cancer Res. 2013 Oct;11(10):1223-34. doi: 10.1158/1541-7786.MCR-13-0252-T. Epub 2013 Jul 15. Mol Cancer Res. 2013. PMID: 23858098 Free PMC article.
-
Genotoxic effects of the water-soluble fraction of heavy oil in the brackish/freshwater amphipod Quadrivisio aff. lutzi (Gammaridea) as assessed using the comet assay.Ecotoxicology. 2013 May;22(4):642-55. doi: 10.1007/s10646-013-1055-z. Epub 2013 Mar 13. Ecotoxicology. 2013. PMID: 23479060
-
DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks.Nat Struct Mol Biol. 2012 Nov;19(11):1084-92. doi: 10.1038/nsmb.2395. Epub 2012 Oct 7. Nat Struct Mol Biol. 2012. PMID: 23042605
-
The Role of Ubiquitination and SUMOylation in Telomere Biology.Curr Issues Mol Biol. 2020;35:85-98. doi: 10.21775/cimb.035.085. Epub 2019 Aug 18. Curr Issues Mol Biol. 2020. PMID: 31422934 Free PMC article. Review.
-
Systematic analysis of alterations in the ubiquitin proteolysis system reveals its contribution to driver mutations in cancer.Nat Cancer. 2020 Jan;1(1):122-135. doi: 10.1038/s43018-019-0001-2. Epub 2019 Dec 2. Nat Cancer. 2020. PMID: 35121836
References
-
- Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin–proteasome system and onto human diseases and drug targeting. Hematology Am. Soc. Hematol. Educ. Program. 2006;1 p. 1–12, 505–6. - PubMed
-
- Ciechanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem. Biophys. Res. Commun. 1978;81(4):1100–1105. - PubMed
-
- Wilkinson K.D., Urban M.K., Haas A.L. Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J. Biol. Chem. 1980;255(16):7529–7532. - PubMed
-
- Hershko A. Components of ubiquitin–protein ligase system, Resolution, affinity purification, and role in protein breakdown. J. Biol. Chem. 1983;258(13):8206–8214. - PubMed
-
- Ciechanover A. “Covalent affinity” purification of ubiquitin-activating enzyme. J. Biol. Chem. 1982;257(5):2537–2542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

