The SNX-PX-BAR family in macropinocytosis: the regulation of macropinosome formation by SNX-PX-BAR proteins
- PMID: 21048941
- PMCID: PMC2966440
- DOI: 10.1371/journal.pone.0013763
The SNX-PX-BAR family in macropinocytosis: the regulation of macropinosome formation by SNX-PX-BAR proteins
Abstract
Background: Macropinocytosis is an actin-driven endocytic process, whereby membrane ruffles fold back onto the plasma membrane to form large (>0.2 µm in diameter) endocytic organelles called macropinosomes. Relative to other endocytic pathways, little is known about the molecular mechanisms involved in macropinocytosis. Recently, members of the Sorting Nexin (SNX) family have been localized to the cell surface and early macropinosomes, and implicated in macropinosome formation. SNX-PX-BAR proteins form a subset of the SNX family and their lipid-binding (PX) and membrane-curvature sensing (BAR) domain architecture further implicates their functional involvement in macropinosome formation.
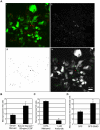
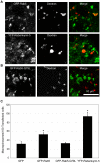
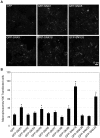
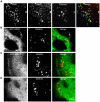
Methodology/principal findings: We exploited the tractability of macropinosomes through image-based screening and systematic overexpression of SNX-PX-BAR proteins to quantitate their effect on macropinosome formation. SNX1 (40.9+/-3.19 macropinosomes), SNX5 (36.99+/-4.48 macropinosomes), SNX9 (37.55+/-2.4 macropinosomes), SNX18 (88.2+/-8 macropinosomes), SNX33 (65.25+/-6.95 macropinosomes) all exhibited statistically significant (p<0.05) increases in average macropinosome numbers per 100 transfected cells as compared to control cells (24.44+/-1.81 macropinosomes). SNX1, SNX5, SNX9, and SNX18 were also found to associate with early-stage macropinosomes within 5 minutes following organelle formation. The modulation of intracellular PI(3,4,5)P(3) levels through overexpression of PTEN or a lipid phosphatase-deficient mutant PTEN(G129E) was also observed to significantly reduce or elevate macropinosome formation respectively; coexpression of PTEN(G129E) with SNX9 or SNX18 synergistically elevated macropinosome formation to 119.4+/-7.13 and 91.4+/-6.37 macropinosomes respectively (p<0.05).
Conclusions/significance: SNX1, SNX5, SNX9, SNX18, and SNX33 were all found to elevate macropinosome formation and (with the exception of SNX33) associate with early-stage macropinosomes. Moreover the effects of SNX9 and SNX18 overexpression in elevating macropinocytosis is likely to be synergistic with the increase in PI(3,4,5)P(3) levels, which is known to accumulate on the cell surface and early-stage macropinocytic cups. Together these findings represent the first systematic functional study into the impact of the SNX-PX-BAR family on macropinocytosis.
Conflict of interest statement
Figures
Similar articles
-
SNX5 is essential for efficient macropinocytosis and antigen processing in primary macrophages.Biol Open. 2012 Sep 15;1(9):904-14. doi: 10.1242/bio.20122204. Epub 2012 Jul 25. Biol Open. 2012. PMID: 23213485 Free PMC article.
-
A role for SNX5 in the regulation of macropinocytosis.BMC Cell Biol. 2008 Oct 14;9:58. doi: 10.1186/1471-2121-9-58. BMC Cell Biol. 2008. PMID: 18854019 Free PMC article.
-
SNX18 is an SNX9 paralog that acts as a membrane tubulator in AP-1-positive endosomal trafficking.J Cell Sci. 2008 May 1;121(Pt 9):1495-505. doi: 10.1242/jcs.028530. Epub 2008 Apr 14. J Cell Sci. 2008. PMID: 18411244
-
Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation.Front Physiol. 2014 Sep 30;5:374. doi: 10.3389/fphys.2014.00374. eCollection 2014. Front Physiol. 2014. PMID: 25324782 Free PMC article. Review.
-
Functional significance of ion channels during macropinosome resolution in immune cells.Front Physiol. 2022 Oct 20;13:1037758. doi: 10.3389/fphys.2022.1037758. eCollection 2022. Front Physiol. 2022. PMID: 36338503 Free PMC article. Review.
Cited by
-
Chlamydia trachomatis TmeA Directly Activates N-WASP To Promote Actin Polymerization and Functions Synergistically with TarP during Invasion.mBio. 2021 Jan 19;12(1):e02861-20. doi: 10.1128/mBio.02861-20. mBio. 2021. PMID: 33468693 Free PMC article.
-
Macropinosome quantitation assay.MethodsX. 2014 Jun 2;1:36-41. doi: 10.1016/j.mex.2014.05.002. eCollection 2014. MethodsX. 2014. PMID: 26150932 Free PMC article.
-
The retromer complex - endosomal protein recycling and beyond.J Cell Sci. 2012 Oct 15;125(Pt 20):4693-702. doi: 10.1242/jcs.103440. Epub 2012 Nov 12. J Cell Sci. 2012. PMID: 23148298 Free PMC article. Review.
-
Activation of NLRP3 Inflammasome by Virus-Like Particles of Human Polyomaviruses in Macrophages.Front Immunol. 2022 Mar 9;13:831815. doi: 10.3389/fimmu.2022.831815. eCollection 2022. Front Immunol. 2022. PMID: 35355981 Free PMC article.
-
Rab8a localisation and activation by Toll-like receptors on macrophage macropinosomes.Philos Trans R Soc Lond B Biol Sci. 2019 Feb 4;374(1765):20180151. doi: 10.1098/rstb.2018.0151. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30966999 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

