The involvement of specific PKC isoenzymes in phorbol ester-mediated regulation of steroidogenic acute regulatory protein expression and steroid synthesis in mouse Leydig cells
- PMID: 21047949
- PMCID: PMC3033061
- DOI: 10.1210/en.2010-0874
The involvement of specific PKC isoenzymes in phorbol ester-mediated regulation of steroidogenic acute regulatory protein expression and steroid synthesis in mouse Leydig cells
Abstract
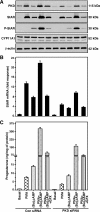
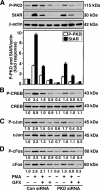
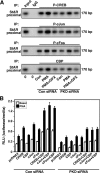
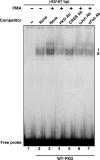
Protein kinase C (PKC) is a multigene family of serine/threonine kinases. PKC is involved in regulating adrenal and gonadal steroidogenesis; however, the functional relevance of the different PKC isoenzymes remains obscure. In this study, we demonstrate that MA-10 mouse Leydig tumor cells express several PKC isoforms to varying levels and that the activation of PKC signaling, by phorbol 12-myristate 13-acetate (PMA) elevated the expression and phosphorylation of PKCα, -δ, -ε, and -μ/protein kinase D (PKD). These responses coincided with the expression of the steroidogenic acute regulatory (StAR) protein and progesterone synthesis. Targeted silencing of PKCα, δ, and ε and PKD, using small interfering RNAs, resulted in deceases in basal and PMA-mediated StAR and steroid levels and demonstrated the importance of PKD in steroidogenesis. PKD was capable of controlling PMA and cAMP/PKA-mediated synergism involved in the steroidogenic response. Further studies pointed out that the regulatory events effected by PKD are associated with cAMP response element-binding protein (CREB) and c-Jun/c-Fos-mediated transcription of the StAR gene. Chromatin immunoprecipitation studies revealed that the activation of phosphorylated CREB, c-Jun, and c-Fos by PMA was correlated with in vivo protein-DNA interactions and the recruitment of CREB-binding protein, whereas knockdown of PKD suppressed the association of these factors with the StAR promoter. Ectopic expression of CREB-binding protein enhanced the trans-activation potential of CREB and c-Jun/c-Fos in StAR gene expression. Using EMSA, a -83/-67-bp region of the StAR promoter was shown to bind PKD-transfected MA-10 nuclear extract in a PMA-responsive manner, targeting CREB and c-Jun/c-Fos proteins. These findings provide evidence for the presence of multiple PKC isoforms and demonstrate the molecular events by which selective isozymes, especially PKD, influence PMA/PKC signaling involved in the regulation of the steroidogenic machinery in mouse Leydig cells.
Figures
Similar articles
-
Basic Leucine Zipper Transcription Factors as Important Regulators of Leydig Cells' Functions.Int J Mol Sci. 2022 Oct 25;23(21):12887. doi: 10.3390/ijms232112887. Int J Mol Sci. 2022. PMID: 36361676 Free PMC article. Review.
-
Mechanisms of protein kinase C signaling in the modulation of 3',5'-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells.Endocrinology. 2009 Jul;150(7):3308-17. doi: 10.1210/en.2008-1668. Epub 2009 Mar 12. Endocrinology. 2009. PMID: 19282384 Free PMC article.
-
The role of JUN in the regulation of PRKCC-mediated STAR expression and steroidogenesis in mouse Leydig cells.J Mol Endocrinol. 2008 Nov;41(5):329-41. doi: 10.1677/JME-08-0077. Epub 2008 Aug 28. J Mol Endocrinol. 2008. PMID: 18755854
-
Role of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 in protein kinase A- and protein kinase C-mediated regulation of the steroidogenic acute regulatory protein expression in mouse Leydig tumor cells: mechanism of action.Endocrinology. 2009 Jan;150(1):187-99. doi: 10.1210/en.2008-0368. Epub 2008 Sep 11. Endocrinology. 2009. PMID: 18787026 Free PMC article.
-
cAMP stimulation of StAR expression and cholesterol metabolism is modulated by co-expression of labile suppressors of transcription and mRNA turnover.Mol Cell Endocrinol. 2011 Apr 10;336(1-2):53-62. doi: 10.1016/j.mce.2010.12.006. Epub 2010 Dec 13. Mol Cell Endocrinol. 2011. PMID: 21147196 Free PMC article. Review.
Cited by
-
Gigantol Improves Cholesterol Metabolism and Progesterone Biosynthesis in MA-10 Leydig Cells.Curr Issues Mol Biol. 2021 Dec 23;44(1):73-93. doi: 10.3390/cimb44010006. Curr Issues Mol Biol. 2021. PMID: 35723385 Free PMC article.
-
Exogenous cholesterol acquisition signaling in LH-responsive MA-10 Leydig cells and in adult mice.J Endocrinol. 2022 Aug 17;254(3):187-199. doi: 10.1530/JOE-22-0043. Print 2022 Sep 1. J Endocrinol. 2022. PMID: 35900012 Free PMC article.
-
Protein kinase C and Src family kinases mediate angiotensin II-induced protein kinase D activation and acute aldosterone production.Mol Cell Endocrinol. 2014 Jul 5;392(1-2):173-81. doi: 10.1016/j.mce.2014.05.015. Epub 2014 May 22. Mol Cell Endocrinol. 2014. PMID: 24859649 Free PMC article.
-
Retinoid regulated macrophage cholesterol efflux involves the steroidogenic acute regulatory protein.Data Brief. 2016 Mar 19;7:940-5. doi: 10.1016/j.dib.2016.03.055. eCollection 2016 Jun. Data Brief. 2016. PMID: 27081671 Free PMC article.
-
Basic Leucine Zipper Transcription Factors as Important Regulators of Leydig Cells' Functions.Int J Mol Sci. 2022 Oct 25;23(21):12887. doi: 10.3390/ijms232112887. Int J Mol Sci. 2022. PMID: 36361676 Free PMC article. Review.
References
-
- Nishizuka Y 1995 Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9:484–496 - PubMed
-
- Peters CA, Maizels ET, Hunzicker-Dunn M 1999 Activation of PKCδ in the rat corpus luteum during pregnancy. Potential role of prolactin signaling. J Biol Chem 274:37499–37505 - PubMed
-
- Parker PJ, Murray-Rust J 2004 PKC at a glance. J Cell Sci 117:131–132 - PubMed
-
- Feng C, Zhang J, Gasana V, Fu W, Liu Y, Zong Z, Yu B 2005 Differential expression of protein kinase C alpha and delta in testes of mouse at various stages of development. Cell Biochem Funct 23:415–420 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

