Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells
- PMID: 21041651
- PMCID: PMC2993354
- DOI: 10.1073/pnas.1009523107
Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells
Abstract
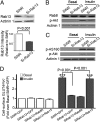
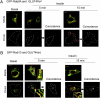
Skeletal muscle is the primary site of dietary glucose disposal, a function that depends on insulin-mediated exocytosis of GLUT4 vesicles to its cell surface. In skeletal muscle and adipocytes, this response involves Akt signaling to the Rab-GAP (GTPase-activating protein) AS160/TBC1D4. Intriguingly, the AS160-targeted Rabs appear to differ, with Rab8A participating in GLUT4 exocytosis in muscle cells and Rab10 in adipocytes, and their activation by insulin is unknown. Rabs 8A, 10, and 13 belong to the same subfamily of Rab-GTPases. Here we show that insulin promotes GTP loading of Rab13 and Rab8A but not Rab10 in rat L6 muscle cells, Rab8A activation preceding that of Rab13. siRNA-mediated Rab13 knockdown blocked the insulin-induced increase of GLUT4 at the muscle cell surface that was rescued by a Rab13 ortholog but not by Rab8A. Constitutively active AS160 lowered basal and insulin-stimulated levels of surface GLUT4, effects that were reversed by overexpressing Rab8A or Rab13, suggesting that both Rabs are targets of AS160-GAP activity in the context of GLUT4 traffic. Rab13 had a broader intracellular distribution compared with the perinuclear restriction of Rab8A, and insulin promoted Rab13 colocalization with GLUT4 at the cell periphery. We conclude that Rab13 and Rab8A are Rab-GTPases activated by insulin, and that downstream of AS160 they regulate traffic of GLUT4 vesicles, possibly acting at distinct steps and sites. These findings close in on the series of events regulating muscle GLUT4 traffic in response to insulin, crucial for whole-body glucose homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

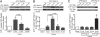
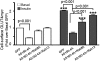
Similar articles
-
Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation.Am J Physiol Cell Physiol. 2008 Oct;295(4):C1016-25. doi: 10.1152/ajpcell.00277.2008. Epub 2008 Aug 13. Am J Physiol Cell Physiol. 2008. PMID: 18701652
-
Myosin Va mediates Rab8A-regulated GLUT4 vesicle exocytosis in insulin-stimulated muscle cells.Mol Biol Cell. 2014 Apr;25(7):1159-70. doi: 10.1091/mbc.E13-08-0493. Epub 2014 Jan 29. Mol Biol Cell. 2014. PMID: 24478457 Free PMC article.
-
Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells.Biochem Biophys Res Commun. 2007 Feb 23;353(4):1074-9. doi: 10.1016/j.bbrc.2006.12.140. Epub 2006 Dec 27. Biochem Biophys Res Commun. 2007. PMID: 17208202
-
Update on GLUT4 Vesicle Traffic: A Cornerstone of Insulin Action.Trends Endocrinol Metab. 2017 Aug;28(8):597-611. doi: 10.1016/j.tem.2017.05.002. Epub 2017 Jun 8. Trends Endocrinol Metab. 2017. PMID: 28602209 Review.
-
Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation.Am J Physiol Cell Physiol. 2014 May 15;306(10):C879-86. doi: 10.1152/ajpcell.00069.2014. Epub 2014 Mar 5. Am J Physiol Cell Physiol. 2014. PMID: 24598362 Review.
Cited by
-
EspH utilizes phosphoinositide and Rab binding domains to interact with plasma membrane infection sites and Rab GTPases.Gut Microbes. 2024 Jan-Dec;16(1):2400575. doi: 10.1080/19490976.2024.2400575. Epub 2024 Sep 23. Gut Microbes. 2024. PMID: 39312647 Free PMC article.
-
Specialized sorting of GLUT4 and its recruitment to the cell surface are independently regulated by distinct Rabs.Mol Biol Cell. 2013 Aug;24(16):2544-57. doi: 10.1091/mbc.E13-02-0103. Epub 2013 Jun 26. Mol Biol Cell. 2013. PMID: 23804653 Free PMC article.
-
Metabolic regulation through the endosomal system.Traffic. 2019 Aug;20(8):552-570. doi: 10.1111/tra.12670. Epub 2019 Jun 24. Traffic. 2019. PMID: 31177593 Free PMC article. Review.
-
Geranylgeranyl pyrophosphate depletion by statins compromises skeletal muscle insulin sensitivity.J Cachexia Sarcopenia Muscle. 2022 Dec;13(6):2697-2711. doi: 10.1002/jcsm.13061. Epub 2022 Aug 12. J Cachexia Sarcopenia Muscle. 2022. PMID: 35961942 Free PMC article.
-
Prior exercise in humans redistributes intramuscular GLUT4 and enhances insulin-stimulated sarcolemmal and endosomal GLUT4 translocation.Mol Metab. 2020 Sep;39:100998. doi: 10.1016/j.molmet.2020.100998. Epub 2020 Apr 17. Mol Metab. 2020. PMID: 32305516 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

