Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L
- PMID: 21041412
- PMCID: PMC2964755
- DOI: 10.1101/gad.1977710
Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L
Abstract
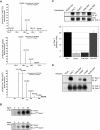
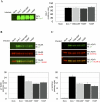
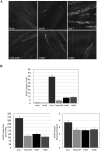
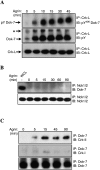
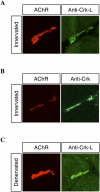
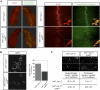
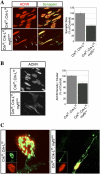
Agrin, released by motor neurons, promotes neuromuscular synapse formation by stimulating MuSK, a receptor tyrosine kinase expressed in skeletal muscle. Phosphorylated MuSK recruits docking protein-7 (Dok-7), an adaptor protein that is expressed selectively in muscle. In the absence of Dok-7, neuromuscular synapses fail to form, and mutations that impair Dok-7 are a major cause of congenital myasthenia in humans. How Dok-7 stimulates synaptic differentiation is poorly understood. Once recruited to MuSK, Dok-7 directly stimulates MuSK kinase activity. This unusual activity of an adapter protein is mediated by the N-terminal region of Dok-7, whereas most mutations that cause congenital myasthenia truncate the C-terminal domain. Here, we demonstrate that Dok-7 also functions downstream from MuSK, and we identify the proteins that are recruited to the C-terminal domain of Dok-7. We show that Agrin stimulates phosphorylation of two tyrosine residues in the C-terminal domain of Dok-7, which leads to recruitment of two adapter proteins: Crk and Crk-L. Furthermore, we show that selective inactivation of Crk and Crk-L in skeletal muscle leads to severe defects in neuromuscular synapses in vivo, revealing a critical role for Crk and Crk-L downstream from Dok-7 in presynaptic and postsynaptic differentiation.
Figures

Similar articles
-
The muscle protein Dok-7 is essential for neuromuscular synaptogenesis.Science. 2006 Jun 23;312(5781):1802-5. doi: 10.1126/science.1127142. Science. 2006. PMID: 16794080
-
Mutations causing DOK7 congenital myasthenia ablate functional motifs in Dok-7.J Biol Chem. 2008 Feb 29;283(9):5518-24. doi: 10.1074/jbc.M708607200. Epub 2007 Dec 29. J Biol Chem. 2008. PMID: 18165682
-
Dok-7 activates the muscle receptor kinase MuSK and shapes synapse formation.Sci Signal. 2009 Feb 24;2(59):ra7. doi: 10.1126/scisignal.2000113. Sci Signal. 2009. PMID: 19244212
-
Structure and activation of MuSK, a receptor tyrosine kinase central to neuromuscular junction formation.Biochim Biophys Acta. 2013 Oct;1834(10):2166-9. doi: 10.1016/j.bbapap.2013.02.034. Epub 2013 Mar 5. Biochim Biophys Acta. 2013. PMID: 23467009 Free PMC article. Review.
-
MuSk function during health and disease.Neurosci Lett. 2020 Jan 18;716:134676. doi: 10.1016/j.neulet.2019.134676. Epub 2019 Dec 4. Neurosci Lett. 2020. PMID: 31811897 Review.
Cited by
-
The role of MuSK in synapse formation and neuromuscular disease.Cold Spring Harb Perspect Biol. 2013 May 1;5(5):a009167. doi: 10.1101/cshperspect.a009167. Cold Spring Harb Perspect Biol. 2013. PMID: 23637281 Free PMC article. Review.
-
The beta-adrenergic agonist salbutamol modulates neuromuscular junction formation in zebrafish models of human myasthenic syndromes.Hum Mol Genet. 2018 May 1;27(9):1556-1564. doi: 10.1093/hmg/ddy062. Hum Mol Genet. 2018. PMID: 29462491 Free PMC article.
-
SRSF1 suppresses selection of intron-distal 5' splice site of DOK7 intron 4 to generate functional full-length Dok-7 protein.Sci Rep. 2017 Sep 5;7(1):10446. doi: 10.1038/s41598-017-11036-z. Sci Rep. 2017. PMID: 28874828 Free PMC article.
-
Two-step release of kinase autoinhibition in discoidin domain receptor 1.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22051-22060. doi: 10.1073/pnas.2007271117. Epub 2020 Aug 24. Proc Natl Acad Sci U S A. 2020. PMID: 32839343 Free PMC article.
-
Sustained Hox5 gene activity is required for respiratory motor neuron development.Nat Neurosci. 2012 Dec;15(12):1636-44. doi: 10.1038/nn.3242. Epub 2012 Oct 28. Nat Neurosci. 2012. PMID: 23103965 Free PMC article.
References
-
- Akakura S, Kar B, Singh S, Cho L, Tibrewal N, Sanokawa-Akakura R, Reichman C, Ravichandran KS, Birge RB 2005. C-terminal SH3 domain of CrkII regulates the assembly and function of the DOCK180/ELMO Rac-GEF. J Cell Physiol 204: 344–351 - PubMed
-
- Ayala R, Shu T, Tsai LH 2007. Trekking across the brain: The journey of neuronal migration. Cell 128: 29–43 - PubMed
-
- Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA 2004. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol 14: 606–610 - PubMed
-
- Beeson D, Higuchi O, Palace J, Cossins J, Spearman H, Maxwell S, Newsom-Davis J, Burke G, Fawcett P, Motomura M, et al. 2006. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 313: 1975–1978 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
