Single-cell optogenetic excitation drives homeostatic synaptic depression
- PMID: 21040851
- PMCID: PMC3111089
- DOI: 10.1016/j.neuron.2010.09.020
Single-cell optogenetic excitation drives homeostatic synaptic depression
Abstract
Homeostatic processes have been proposed to explain the discrepancy between the dynamics of synaptic plasticity and the stability of brain function. Forms of synaptic plasticity such as long-term potentiation alter synaptic activity in a synapse- and cell-specific fashion. Although network-wide excitation triggers compensatory homeostatic changes, it is unknown whether neurons initiate homeostatic synaptic changes in response to cell-autonomous increases in excitation. Here we employ optogenetic tools to cell-autonomously excite CA1 pyramidal neurons and find that a compensatory postsynaptic depression of both AMPAR and NMDAR function results. Elevated calcium influx through L-type calcium channels leads to activation of a pathway involving CaM kinase kinase and CaM kinase 4 that induces synaptic depression of AMPAR and NMDAR responses. The synaptic depression of AMPARs but not of NMDARs requires protein synthesis and the GluA2 AMPAR subunit, indicating that downstream of CaM kinase activation divergent pathways regulate homeostatic AMPAR and NMDAR depression.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
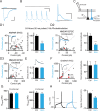

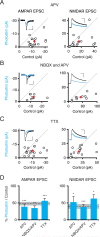


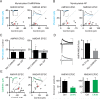
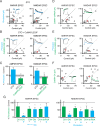
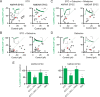
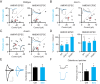
Comment in
-
Homeostatic plasticity: single hippocampal neurons see the light.Neuron. 2010 Nov 4;68(3):326-8. doi: 10.1016/j.neuron.2010.10.025. Neuron. 2010. PMID: 21040836
-
Plasticity: Don't get too excited!Nat Rev Neurosci. 2011 Jan;12(1):6. doi: 10.1038/nrn2966. Nat Rev Neurosci. 2011. PMID: 21218573 No abstract available.
Similar articles
-
Control of Homeostatic Synaptic Plasticity by AKAP-Anchored Kinase and Phosphatase Regulation of Ca2+-Permeable AMPA Receptors.J Neurosci. 2018 Mar 14;38(11):2863-2876. doi: 10.1523/JNEUROSCI.2362-17.2018. Epub 2018 Feb 13. J Neurosci. 2018. PMID: 29440558 Free PMC article.
-
Control of Excitation/Inhibition Balance in a Hippocampal Circuit by Calcium Sensor Protein Regulation of Presynaptic Calcium Channels.J Neurosci. 2018 May 2;38(18):4430-4440. doi: 10.1523/JNEUROSCI.0022-18.2018. Epub 2018 Apr 13. J Neurosci. 2018. PMID: 29654190 Free PMC article.
-
Calcium-calmodulin signalling pathway up-regulates glutamatergic synaptic function in non-pyramidal, fast spiking rat hippocampal CA1 neurons.J Physiol. 2001 Jun 1;533(Pt 2):407-22. doi: 10.1111/j.1469-7793.2001.0407a.x. J Physiol. 2001. PMID: 11389201 Free PMC article.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
The AMPA Receptor Code of Synaptic Plasticity.Neuron. 2018 Oct 24;100(2):314-329. doi: 10.1016/j.neuron.2018.10.018. Neuron. 2018. PMID: 30359599 Free PMC article. Review.
Cited by
-
Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity.J Neurosci. 2013 May 15;33(20):8810-9. doi: 10.1523/JNEUROSCI.4502-12.2013. J Neurosci. 2013. PMID: 23678123 Free PMC article.
-
The bimodal mechanism of interaction between dopamine and mitochondria as reflected in Parkinson's disease and in schizophrenia.J Neural Transm (Vienna). 2020 Feb;127(2):159-168. doi: 10.1007/s00702-019-02120-x. Epub 2019 Dec 17. J Neural Transm (Vienna). 2020. PMID: 31848775 Review.
-
Potentiation of synaptic AMPA receptors induced by the deletion of NMDA receptors requires the GluA2 subunit.J Neurophysiol. 2011 Feb;105(2):923-8. doi: 10.1152/jn.00725.2010. Epub 2010 Oct 27. J Neurophysiol. 2011. PMID: 20980546 Free PMC article.
-
NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14765-70. doi: 10.1073/pnas.1314137110. Epub 2013 Aug 20. Proc Natl Acad Sci U S A. 2013. PMID: 23964123 Free PMC article.
-
Cornichons control ER export of AMPA receptors to regulate synaptic excitability.Neuron. 2013 Oct 2;80(1):129-42. doi: 10.1016/j.neuron.2013.07.028. Epub 2013 Oct 2. Neuron. 2013. PMID: 24094107 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

