A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice
- PMID: 20980993
- PMCID: PMC3021093
- DOI: 10.1038/npp.2010.186
A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice
Abstract
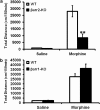
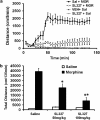
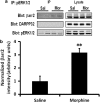
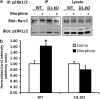
Morphine is a widely used analgesic in humans that is associated with multiple untoward effects, such as addiction and physical dependence. In rodent models, morphine also induces locomotor activity. These effects likely involve functionally selective mechanisms. Indeed, G protein-coupled receptor desensitization and adaptor protein β-arrestin 2 (βarr2) through its interaction with the μ-opioid receptor regulates the analgesic but not the rewarding properties of morphine. However, βarr2 is also required for morphine-induced locomotor activity in mice, but the exact cellular and molecular mechanisms that mediate this arrestin-dependent behavior are not understood. In this study, we show that βarr2 is required for morphine-induced locomotor activity in a dopamine D1 receptor (D1R)-dependent manner and that a βarr2/phospho-ERK (βarr2/pERK) signaling complex may mediate this behavior. Systemic administration of SL327, an MEK inhibitor, inhibits morphine-induced locomotion in wild-type mice in a dose-dependent manner. Acute morphine administration to mice promotes the formation of a βarr2/pERK signaling complex. Morphine-induced locomotor activity and formation of the βarr2/pERK signaling complex is blunted in D1R knockout (D1-KO) mice and is presumably independent of D2 dopamine receptors. However, D1Rs are not required for morphine-induced reward as D1-KO mice show the same conditioned place preference for morphine as do control mice. Taken together, these results suggest a potential role for a D1R-dependent βarr2/pERK signaling complex in selectively mediating the locomotor-stimulating but not the rewarding properties of morphine.
Figures
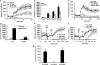
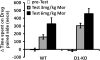
Similar articles
-
Loss of β-arrestin2 in D2 cells alters neuronal excitability in the nucleus accumbens and behavioral responses to psychostimulants and opioids.Addict Biol. 2020 Nov;25(6):e12823. doi: 10.1111/adb.12823. Epub 2019 Aug 23. Addict Biol. 2020. PMID: 31441201 Free PMC article.
-
Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice.J Neurosci. 2003 Nov 12;23(32):10265-73. doi: 10.1523/JNEUROSCI.23-32-10265.2003. J Neurosci. 2003. PMID: 14614085 Free PMC article.
-
Activation of the cAMP/PKA/DARPP-32 signaling pathway is required for morphine psychomotor stimulation but not for morphine reward.Neuropsychopharmacology. 2007 Sep;32(9):1995-2003. doi: 10.1038/sj.npp.1301321. Epub 2007 Jan 24. Neuropsychopharmacology. 2007. PMID: 17251906
-
A behavioral genetics approach to understanding D1 receptor involvement in phasic dopamine signaling.Mol Cell Neurosci. 2011 Jan;46(1):21-31. doi: 10.1016/j.mcn.2010.09.011. Epub 2010 Oct 1. Mol Cell Neurosci. 2011. PMID: 20888914 Free PMC article. Review.
-
Functional Selectivity of Dopamine D1 Receptor Signaling: Retrospect and Prospect.Int J Mol Sci. 2021 Nov 3;22(21):11914. doi: 10.3390/ijms222111914. Int J Mol Sci. 2021. PMID: 34769344 Free PMC article. Review.
Cited by
-
Identification of G protein-biased agonists that fail to recruit β-arrestin or promote internalization of the D1 dopamine receptor.ACS Chem Neurosci. 2015 Apr 15;6(4):681-92. doi: 10.1021/acschemneuro.5b00020. Epub 2015 Feb 20. ACS Chem Neurosci. 2015. PMID: 25660762 Free PMC article.
-
D1 dopamine receptors intrinsic activity and functional selectivity affect working memory in prefrontal cortex.Mol Psychiatry. 2021 Feb;26(2):645-655. doi: 10.1038/s41380-018-0312-1. Epub 2018 Dec 7. Mol Psychiatry. 2021. PMID: 30532019 Free PMC article.
-
α-[Amino(4-aminophenyl)thio]methylene-2-(trifluoromethyl)benzeneacetonitrile; Configurational equilibria in solution.Bioorg Chem. 2021 Aug;113:104955. doi: 10.1016/j.bioorg.2021.104955. Epub 2021 May 4. Bioorg Chem. 2021. PMID: 34034134 Free PMC article.
-
Targeting β-arrestin2 in the treatment of L-DOPA-induced dyskinesia in Parkinson's disease.Proc Natl Acad Sci U S A. 2015 May 12;112(19):E2517-26. doi: 10.1073/pnas.1502740112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918399 Free PMC article.
-
Toward Directing Opioid Receptor Signaling to Refine Opioid Therapeutics.Biol Psychiatry. 2020 Jan 1;87(1):15-21. doi: 10.1016/j.biopsych.2019.10.020. Epub 2019 Oct 31. Biol Psychiatry. 2020. PMID: 31806082 Free PMC article. Review.
References
-
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. - PubMed
-
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. - PubMed
-
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080. - PubMed
-
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

