A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases
- PMID: 20978148
- PMCID: PMC2970766
- DOI: 10.1158/1078-0432.CCR-10-0791
A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases
Abstract
Purpose: To determine the maximum tolerated dose (MTD), toxicity spectrum, clinical activity, and biological effects of the tropism-modified, infectivity-enhanced conditionally replicative adenovirus (CRAd), Ad5-Δ24-Arg-Gly-Asp (RGD), in patients with malignant gynecologic diseases.
Experimental design: Cohorts of eligible patients were treated daily for 3 days through an i.p. catheter. Vector doses ranged from 1 × 10(9) to 1 × 10(12) viral particles per day. Toxicity was evaluated using CTCv3.0. CA-125 and Response Evaluation Criteria in Solid Tumors (RECIST) criteria were used to determine clinical efficacy. Corollary biological studies included assessment of CRAd replication, wild-type virus generation, viral shedding, and neutralizing antibody response.
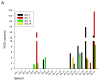
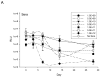
Results: Twenty-one patients were treated. Adverse clinical effects were limited to grade 1/2 fever, fatigue, or abdominal pain. No vector-related grade 3/4 toxicities were noted. No clinically significant laboratory abnormalities were noted. The maximum tolerated dose was not reached. Over a 1 month follow-up, 15 (71%) patients had stable disease and six (29%) had progressive disease. No partial or complete responses were noted. Seven patients had a decrease in CA-125; four had a >20% drop. RGD-specific PCR showed the presence of study vector in ascites of 16 patients. Seven revealed an increase in virus after day 3, suggesting replication of Ad5-Δ24-RGD. Minimal wild-type virus generation was detected. Viral shedding studies showed insignificant shedding in the serum, saliva, and urine. Anti-adenoviral neutralizing antibody effects were prevalent.
Conclusions: This study, the first to evaluate an infectivity-enhanced CRAd in human cancer, shows the feasibility, safety, potential antitumor response, and biological activity of this approach in ovarian cancer. Further evaluation of infectivity enhanced virotherapy approaches for malignant gynecologic diseases is warranted.
©2010 AACR.
Figures
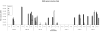
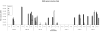



Similar articles
-
A phase I clinical trial of Ad5/3-Δ24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer.Gynecol Oncol. 2013 Sep;130(3):518-24. doi: 10.1016/j.ygyno.2013.06.003. Epub 2013 Jun 10. Gynecol Oncol. 2013. PMID: 23756180 Free PMC article. Clinical Trial.
-
A new generation of serotype chimeric infectivity-enhanced conditionally replicative adenovirals: the safety profile of ad5/3-Δ24 in advance of a phase I clinical trial in ovarian cancer patients.Hum Gene Ther. 2011 Jul;22(7):821-8. doi: 10.1089/hum.2010.180. Epub 2011 Mar 23. Hum Gene Ther. 2011. PMID: 21171861 Free PMC article.
-
A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer.Clin Cancer Res. 2012 Jun 15;18(12):3440-51. doi: 10.1158/1078-0432.CCR-11-2852. Epub 2012 Apr 17. Clin Cancer Res. 2012. PMID: 22510347 Free PMC article. Clinical Trial.
-
Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-delta24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer.Am J Obstet Gynecol. 2007 Apr;196(4):389.e1-9; discussion 389.e9-10. doi: 10.1016/j.ajog.2006.12.016. Am J Obstet Gynecol. 2007. PMID: 17403430
-
Targeted and shielded adenovectors for cancer therapy.Cancer Immunol Immunother. 2006 Nov;55(11):1412-9. doi: 10.1007/s00262-006-0158-2. Epub 2006 Apr 13. Cancer Immunol Immunother. 2006. PMID: 16612598 Free PMC article. Review.
Cited by
-
Targeted Adenoviral Vector Demonstrates Enhanced Efficacy for In Vivo Gene Therapy of Uterine Leiomyoma.Reprod Sci. 2016 Apr;23(4):464-74. doi: 10.1177/1933719116630413. Epub 2016 Feb 16. Reprod Sci. 2016. PMID: 26884457 Free PMC article.
-
Challenges and strategies toward oncolytic virotherapy for leptomeningeal metastasis.J Transl Med. 2024 Nov 5;22(1):1000. doi: 10.1186/s12967-024-05794-4. J Transl Med. 2024. PMID: 39501324 Free PMC article. Review.
-
Targeting adenoviral vectors for enhanced gene therapy of uterine leiomyomas.Hum Reprod. 2013 Sep;28(9):2398-406. doi: 10.1093/humrep/det275. Epub 2013 Jul 2. Hum Reprod. 2013. PMID: 23820419 Free PMC article.
-
Paclitaxel resistance increases oncolytic adenovirus efficacy via upregulated CAR expression and dysfunctional cell cycle control.Mol Oncol. 2015 Apr;9(4):791-805. doi: 10.1016/j.molonc.2014.12.007. Epub 2014 Dec 29. Mol Oncol. 2015. PMID: 25560085 Free PMC article.
-
Oncolytic adenoviruses targeted to Human Papilloma Virus-positive head and neck squamous cell carcinomas.Oral Oncol. 2016 May;56:25-31. doi: 10.1016/j.oraloncology.2016.02.014. Epub 2016 Mar 18. Oral Oncol. 2016. PMID: 27086483 Free PMC article.
References
-
- Brinton LA, Lacey JV, Sherman ME. Epidemiology of Gynecologic Cancers. In: Hoskins WJ, Perez CJ, Young RC, et al., editors. Principles and practice of gynecologic oncology. 4th. Philadelphia, PA: Lippincott-Raven; 2005. pp. 3–32.
-
- Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract. 2007;4:101–17. - PubMed
-
- Kimball KJ, Numnum TM, Rocconi RP, Alvarez RD. Gene therapy for ovarian cancer. Curr Oncol Rep. 2006;6:441–7. - PubMed
-
- Alemany R, Balague C, Curiel D. Replicative adenovirus for cancer therapy. Nature Biotechnol. 2000;18:723–7. - PubMed
-
- Agarwal R, Linch M, Kaye SB. Novel therapeutic agents in ovarian cancer. Eur J Surg Oncol. 2006;32:875–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

