Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection
- PMID: 20967271
- PMCID: PMC2953504
- DOI: 10.1371/journal.pone.0013310
Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection
Abstract
Background: Although combination antiretroviral therapy (cART) initiated in the acute phase of HIV-1 infection may prevent expansion of the latent reservoir, its benefits remain controversial. In the current study, HIV-1 RNA transcription patterns in peripheral blood mononuclear cells (PBMC) were monitored during acute cART to assess the effect of early treatment on cellular viral reservoirs.
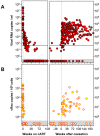
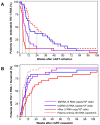
Methodology/principal findings: Acutely HIV-1 infected patients (n = 24) were treated within 3-15 weeks after infection. Patients elected to cease treatment after ≥1 year of therapy. HIV-1 DNA (vDNA), HIV-1 RNA species expressed both in latently and productively infected cells, unspliced (UsRNA), multiply spliced (MsRNA-tatrev; MsRNA-nef), and PBMC-associated extracellular virion RNA (vRex), expressed specifically by productively infected cells, were quantified in PBMC by patient matched real-time PCR prior, during and post cART. In a matched control-group of patients on successful cART started during chronic infection (n = 15), UsRNA in PBMC and vDNA were measured cross-sectionally. In contrast to previous reports, PBMC-associated HIV-1 RNAs declined to predominantly undetectable levels on cART. After cART cessation, UsRNA, vRex, and MsRNA-tatrev rebounded to levels not significantly different to those at baseline (p>0.1). In contrast, MsRNA-nef remained significantly lower as compared to pretreatment (p = 0.015). UsRNA expressed at the highest levels of all viral RNAs, was detectable on cART in 42% of patients with cART initiated during acute infection as opposed to 87% of patients on cART initiated during chronic infection (Fisher's exact test; p = 0.008). Accordingly, UsRNA levels were 105-fold lower in the acute as compared to the chronic group.
Conclusion: Early intervention resulted in profound depletion of PBMC expressing HIV-1 RNA. This is contrary to chronically infected patients who predominantly showed continuous UsRNA expression on cART. Thus, antiretroviral treatment initiated during the acute phase of infection prevented establishment or expansion of long-lived transcriptionally active viral cellular reservoirs in peripheral blood.
Conflict of interest statement
Figures



Similar articles
-
Cellular levels of HIV unspliced RNA from patients on combination antiretroviral therapy with undetectable plasma viremia predict the therapy outcome.PLoS One. 2009 Dec 31;4(12):e8490. doi: 10.1371/journal.pone.0008490. PLoS One. 2009. PMID: 20046870 Free PMC article.
-
Cell-Associated HIV-1 DNA and RNA Decay Dynamics During Early Combination Antiretroviral Therapy in HIV-1-Infected Infants.Clin Infect Dis. 2015 Dec 15;61(12):1862-70. doi: 10.1093/cid/civ688. Epub 2015 Aug 13. Clin Infect Dis. 2015. PMID: 26270687 Free PMC article.
-
Biphasic decay kinetics suggest progressive slowing in turnover of latently HIV-1 infected cells during antiretroviral therapy.Retrovirology. 2008 Nov 26;5:107. doi: 10.1186/1742-4690-5-107. Retrovirology. 2008. PMID: 19036147 Free PMC article.
-
Early Combination Antiretroviral Therapy Limits HIV-1 Persistence in Children.Annu Rev Med. 2016;67:201-13. doi: 10.1146/annurev-med-091114-111159. Annu Rev Med. 2016. PMID: 26768239 Review.
-
Changes in HIV reservoirs during long-term antiretroviral therapy.Curr Opin HIV AIDS. 2015 Jan;10(1):43-8. doi: 10.1097/COH.0000000000000119. Curr Opin HIV AIDS. 2015. PMID: 25402706 Free PMC article. Review.
Cited by
-
Fixed-dose combination emtricitabine/tenofovir/efavirenz initiated during acute HIV infection; 96-week efficacy and durability.AIDS. 2016 Nov 28;30(18):2815-2822. doi: 10.1097/QAD.0000000000001255. AIDS. 2016. PMID: 27662549 Free PMC article.
-
No evidence of posttreatment control after early initiation of antiretroviral therapy.AIDS. 2015 Oct 23;29(16):2093-7. doi: 10.1097/QAD.0000000000000816. AIDS. 2015. PMID: 26544575 Free PMC article.
-
Short-course antiretroviral therapy in primary HIV infection.N Engl J Med. 2013 Jan 17;368(3):207-17. doi: 10.1056/NEJMoa1110039. N Engl J Med. 2013. PMID: 23323897 Free PMC article. Clinical Trial.
-
Diagnostic performance of line-immunoassay based algorithms for incident HIV-1 infection.BMC Infect Dis. 2012 Apr 12;12:88. doi: 10.1186/1471-2334-12-88. BMC Infect Dis. 2012. PMID: 22497961 Free PMC article.
-
Cohort Profile: The Zurich Primary HIV Infection Study.Microorganisms. 2024 Jan 31;12(2):302. doi: 10.3390/microorganisms12020302. Microorganisms. 2024. PMID: 38399706 Free PMC article.
References
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. - PubMed
-
- Opravil M, Cone RW, Fischer M, Vernazza P, Basseti S, et al. Effects of early antiretroviral treatment on HIV-1 RNA in blood and lymphoid tissue: A randomized trial of double versus triple therapy. J Acquir Immune Defic Syndr Hum Retrovirol. 2000;23:17–25. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. - PubMed
-
- Stebbing J, Gazzard B, Douek DC. Where does HIV live? N Engl J Med. 2004;350:1872–1880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

