Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson's disease
- PMID: 20959812
- PMCID: PMC3017447
- DOI: 10.1038/mt.2010.217
Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson's disease
Abstract
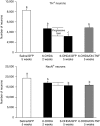
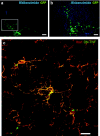
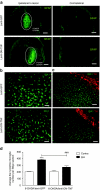
Parkinson's disease (PD) is a progressive neurodegenerative disorder typified by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). Recent evidence indicates that neuroinflammation may play a critical role in the pathogenesis of PD, particularly tumor necrosis factor (TNF). We have previously shown that soluble TNF (solTNF) is required to mediate robust degeneration induced by 6-hydroxydopamine (6-OHDA) or lipopolysaccharide. What remains unknown is whether TNF inhibition can attenuate the delayed and progressive phase of neurodegeneration. To test this, rats were injected in the SNpc with lentivirus encoding dominant-negative TNF (lenti-DN-TNF) 2 weeks after receiving a 6-OHDA lesion. Remarkably, when examined 5 weeks after the initial 6-OHDA lesion, no further loss of nigral DA neurons was observed. Lenti-DN-TNF also attenuated microglial activation. Together, these data suggest that TNF is likely a critical mediator of nigral DA neuron death during the delayed and progressive phase of neurodegeneration, and that microglia may be the principal cell type involved. These promising findings provide compelling reasons to perform DN-TNF gene transfer studies in nonhuman primates with the long-term goal of using it in the clinic to prevent the delayed and progressive degeneration of DA neurons that gives rise to motor symptoms in PD.
Figures


Similar articles
-
Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease.J Neurosci. 2006 Sep 13;26(37):9365-75. doi: 10.1523/JNEUROSCI.1504-06.2006. J Neurosci. 2006. PMID: 16971520 Free PMC article.
-
Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats.Mol Ther. 2008 Sep;16(9):1572-9. doi: 10.1038/mt.2008.146. Epub 2008 Jul 15. Mol Ther. 2008. PMID: 18628756 Free PMC article.
-
Peripheral administration of the selective inhibitor of soluble tumor necrosis factor (TNF) XPro®1595 attenuates nigral cell loss and glial activation in 6-OHDA hemiparkinsonian rats.J Parkinsons Dis. 2014;4(3):349-60. doi: 10.3233/JPD-140410. J Parkinsons Dis. 2014. PMID: 25061061 Free PMC article.
-
Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review.
-
A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson's disease and potential molecular targets.Exp Neurol. 2014 Jun;256:126-32. doi: 10.1016/j.expneurol.2013.05.014. Epub 2013 May 28. Exp Neurol. 2014. PMID: 23726958 Free PMC article. Review.
Cited by
-
Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease.J Parkinsons Dis. 2015;5(1):1-19. doi: 10.3233/JPD-140491. J Parkinsons Dis. 2015. PMID: 25588354 Free PMC article. Review.
-
LRRK2 inhibition attenuates microglial inflammatory responses.J Neurosci. 2012 Feb 1;32(5):1602-11. doi: 10.1523/JNEUROSCI.5601-11.2012. J Neurosci. 2012. PMID: 22302802 Free PMC article.
-
Etanercept, a widely used inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal ganglion cell loss in a rat model of glaucoma.PLoS One. 2012;7(7):e40065. doi: 10.1371/journal.pone.0040065. Epub 2012 Jul 3. PLoS One. 2012. PMID: 22802951 Free PMC article.
-
Cold-inducible protein RBM3 mediates hypothermic neuroprotection against neurotoxin rotenone via inhibition on MAPK signalling.J Cell Mol Med. 2019 Oct;23(10):7010-7020. doi: 10.1111/jcmm.14588. Epub 2019 Aug 22. J Cell Mol Med. 2019. PMID: 31436914 Free PMC article.
-
Aspirin protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity in primary midbrain cultures.J Mol Neurosci. 2012 Jan;46(1):153-61. doi: 10.1007/s12031-011-9541-3. Epub 2011 May 17. J Mol Neurosci. 2012. PMID: 21584653
References
-
- McGeer PL., and, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. - PubMed
-
- Aggarwal BB, Samanta A, Feldmann M.2000. TNFα. In: Oppenheim JJ, Feldman M (eds) Cytokine Reference. Academic: New York. 414–434.
-
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y., and, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. - PubMed
-
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K., and, Nagatsu T. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

