Agonist activation of the G protein-coupled receptor GPR35 involves transmembrane domain III and is transduced via Gα₁₃ and β-arrestin-2
- PMID: 20958291
- PMCID: PMC3041261
- DOI: 10.1111/j.1476-5381.2010.01082.x
Agonist activation of the G protein-coupled receptor GPR35 involves transmembrane domain III and is transduced via Gα₁₃ and β-arrestin-2
Abstract
Background and purpose: GPR35 is a poorly characterized G protein-coupled receptor at which kynurenic acid has been suggested to be the endogenous ligand. We wished to test this and develop assays appropriate for the study of this receptor.
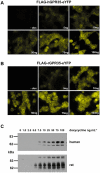
Experimental approach: Human and rat orthologues of GPR35 were engineered and expressed and assays developed to assess interaction with β-arrestin-2, activation of Gα₁₃ and agonist-induced internalization.
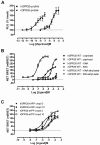
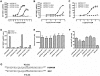
Key results: GPR35-β-arrestin-2 interaction assays confirmed that both the endogenous tryptophan metabolite kynurenic acid and the synthetic ligand zaprinast had agonist action at each orthologue. Zaprinast was substantially more potent than kynurenic acid at each and both agonists displayed substantially greater potency at rat GPR35. Two novel thiazolidinediones also displayed agonism and displayed similar potency at each GPR35 orthologue. The three ligand classes acted orthosterically with respect to each other, suggesting overlapping binding sites and, consistent with this, mutation to alanine of the conserved arginine at position 3.36 or tyrosine 3.32 in transmembrane domain III abolished β-arrestin-2 recruitment in response to each ligand at each orthologue.
Conclusions and implications: These studies indicate that β-arrestin-2 interaction assays are highly appropriate to explore the pharmacology of GPR35 and that Gα₁₃ activation is an alternative avenue of signal generation from GPR35. Arginine and tyrosine residues in transmembrane domain III are integral to agonist recognition and function of this receptor. The potency of kynurenic acid at human GPR35 is sufficiently low, however, to question whether it is likely to be the true endogenous ligand for this receptor.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
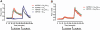


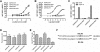
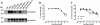
Similar articles
-
Identification of novel species-selective agonists of the G-protein-coupled receptor GPR35 that promote recruitment of β-arrestin-2 and activate Gα13.Biochem J. 2010 Dec 15;432(3):451-9. doi: 10.1042/BJ20101287. Biochem J. 2010. PMID: 20919992
-
G-protein coupled receptor 35 (GPR35) activation and inflammatory pain: Studies on the antinociceptive effects of kynurenic acid and zaprinast.Neuropharmacology. 2011 Jun;60(7-8):1227-31. doi: 10.1016/j.neuropharm.2010.11.014. Epub 2010 Nov 24. Neuropharmacology. 2011. PMID: 21110987
-
Antagonists of GPR35 display high species ortholog selectivity and varying modes of action.J Pharmacol Exp Ther. 2012 Dec;343(3):683-95. doi: 10.1124/jpet.112.198945. Epub 2012 Sep 11. J Pharmacol Exp Ther. 2012. PMID: 22967846 Free PMC article.
-
G protein-independent cell-based assays for drug discovery on seven-transmembrane receptors.Biotechnol Annu Rev. 2008;14:253-74. doi: 10.1016/S1387-2656(08)00010-0. Biotechnol Annu Rev. 2008. PMID: 18606367 Review.
-
Recent advances in GPR35 pharmacology; 5-HIAA serotonin metabolite becomes a ligand.Arch Pharm Res. 2023 Jun;46(6):550-563. doi: 10.1007/s12272-023-01449-y. Epub 2023 May 25. Arch Pharm Res. 2023. PMID: 37227682 Review.
Cited by
-
Insights into divalent cation regulation and G13-coupling of orphan receptor GPR35.Cell Discov. 2022 Dec 21;8(1):135. doi: 10.1038/s41421-022-00499-8. Cell Discov. 2022. PMID: 36543774 Free PMC article.
-
G-protein coupled receptor 35 (GPR35) regulates the colonic epithelial cell response to enterotoxigenic Bacteroides fragilis.Commun Biol. 2021 May 14;4(1):585. doi: 10.1038/s42003-021-02014-3. Commun Biol. 2021. PMID: 33990686 Free PMC article.
-
Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery.J Neural Transm (Vienna). 2012 Feb;119(2):133-9. doi: 10.1007/s00702-011-0763-x. Epub 2012 Jan 4. J Neural Transm (Vienna). 2012. PMID: 22215208 Review.
-
Mitochondrial remodeling and ischemic protection by G protein-coupled receptor 35 agonists.Science. 2022 Aug 5;377(6606):621-629. doi: 10.1126/science.abm1638. Epub 2022 Aug 4. Science. 2022. PMID: 35926043 Free PMC article.
-
G protein-coupled receptors not currently in the spotlight: free fatty acid receptor 2 and GPR35.Br J Pharmacol. 2018 Jul;175(13):2543-2553. doi: 10.1111/bph.14042. Epub 2017 Nov 2. Br J Pharmacol. 2018. PMID: 28940377 Free PMC article. Review.
References
-
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428.
-
- Burt AR, Carr IC, Mullaney I, Anderson NG, Milligan G. Agonist activation of p42 and p44 mitogen-activated protein kinases following expression of the mouse delta opioid receptor in Rat-1 fibroblasts: effects of receptor expression levels and comparisons with G-protein activation. Biochem J. 1996;320:227–235. - PMC - PubMed
-
- Canals M, Milligan G. Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed Mu opioid receptors. J Biol Chem. 2008;283:11424–11434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

