IL-35-mediated induction of a potent regulatory T cell population
- PMID: 20953201
- PMCID: PMC3008395
- DOI: 10.1038/ni.1952
IL-35-mediated induction of a potent regulatory T cell population
Abstract
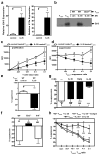
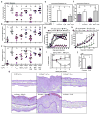
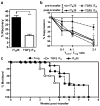
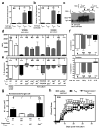
Regulatory T cells (T(reg) cells) have a critical role in the maintenance of immunological self-tolerance. Here we show that treatment of naive human or mouse T cells with IL-35 induced a regulatory population, which we call 'iT(R)35 cells', that mediated suppression via IL-35 but not via the inhibitory cytokines IL-10 or transforming growth factor-β (TGF-β). We found that iT(R)35 cells did not express or require the transcription factor Foxp3, and were strongly suppressive and stable in vivo. T(reg) cells induced the generation of iT(R)35 cells in an IL-35- and IL-10-dependent manner in vitro and induced their generation in vivo under inflammatory conditions in intestines infected with Trichuris muris and within the tumor microenvironment (B16 melanoma and MC38 colorectal adenocarcinoma), where they contributed to the regulatory milieu. Thus, iT(R)35 cells constitute a key mediator of infectious tolerance and contribute to T(reg) cell-mediated tumor progression. Furthermore, iT(R)35 cells generated ex vivo might have therapeutic utility.
Conflict of interest statement
The authors declare competing financial interests.
Figures


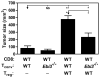
Comment in
-
Regulatory ripples.Nat Immunol. 2010 Dec;11(12):1077-8. doi: 10.1038/ni1210-1077. Nat Immunol. 2010. PMID: 21079629 Free PMC article. Review.
Similar articles
-
IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells.Eur J Immunol. 2007 Nov;37(11):3155-63. doi: 10.1002/eji.200737766. Eur J Immunol. 2007. PMID: 17918200
-
Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance.J Immunol. 2011 Jun 15;186(12):6661-6. doi: 10.4049/jimmunol.1100315. Epub 2011 May 16. J Immunol. 2011. Retraction in: J Immunol. 2013 Aug 15;191(4):2018. doi: 10.4049/jimmunol.1390040. PMID: 21576509 Free PMC article. Retracted.
-
Expression of tumor necrosis factor-α induced protein 8 like-2 contributes to the immunosuppressive property of CD4(+)CD25(+) regulatory T cells in mice.Mol Immunol. 2011 Oct;49(1-2):219-26. doi: 10.1016/j.molimm.2011.08.016. Epub 2011 Oct 2. Mol Immunol. 2011. PMID: 21963221
-
FOXP3+ regulatory T cells in the human immune system.Nat Rev Immunol. 2010 Jul;10(7):490-500. doi: 10.1038/nri2785. Epub 2010 Jun 18. Nat Rev Immunol. 2010. PMID: 20559327 Review.
-
T regulatory cells in allergy and health: a question of allergen specificity and balance.Int Arch Allergy Immunol. 2004 Sep;135(1):73-82. doi: 10.1159/000080523. Epub 2004 Aug 30. Int Arch Allergy Immunol. 2004. PMID: 15340251 Review.
Cited by
-
"Complimenting the Complement": Mechanistic Insights and Opportunities for Therapeutics in Hepatocellular Carcinoma.Front Oncol. 2021 Feb 24;10:627701. doi: 10.3389/fonc.2020.627701. eCollection 2020. Front Oncol. 2021. PMID: 33718121 Free PMC article. Review.
-
Interleukin-35: Expanding Its Job Profile.J Interferon Cytokine Res. 2015 Jul;35(7):499-512. doi: 10.1089/jir.2015.0015. Epub 2015 Apr 28. J Interferon Cytokine Res. 2015. PMID: 25919641 Free PMC article. Review.
-
B cell-T cell interplay in immune regulation: A focus on follicular regulatory T and regulatory B cell functions.Front Cell Dev Biol. 2022 Sep 23;10:991840. doi: 10.3389/fcell.2022.991840. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36211467 Free PMC article. Review.
-
Distinct subunit pairing criteria within the heterodimeric IL-12 cytokine family.Mol Immunol. 2012 Jun;51(2):234-44. doi: 10.1016/j.molimm.2012.03.025. Epub 2012 Apr 7. Mol Immunol. 2012. PMID: 22487722 Free PMC article.
-
Cytokine-modulating strategies and newer cytokine targets for arthritis therapy.Int J Mol Sci. 2014 Dec 31;16(1):887-906. doi: 10.3390/ijms16010887. Int J Mol Sci. 2014. PMID: 25561237 Free PMC article. Review.
References
-
- Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. - PubMed
-
- Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. - PubMed
-
- Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–313. - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- R01 AI039480/AI/NIAID NIH HHS/United States
- F32 AI072816/AI/NIAID NIH HHS/United States
- R01 AI074878-04/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- R01 AI061570-08/AI/NIAID NIH HHS/United States
- R01 AI091977/AI/NIAID NIH HHS/United States
- R01 AI039480-15/AI/NIAID NIH HHS/United States
- R01 AI091977-01/AI/NIAID NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- P30 CA021765-31/CA/NCI NIH HHS/United States
- R01 CA203689/CA/NCI NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- F32 AI072816-03/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

