Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2
- PMID: 20951774
- PMCID: PMC2991419
- DOI: 10.1016/j.neuroscience.2010.10.027
Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2
Abstract
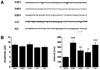
Inheritance of the APOE4 allele is a well established genetic risk factor linked to the development of late onset Alzheimer's disease. As the major lipid transport protein in the central nervous system, apolipoprotein (apo) E plays an important role in the assembly and maintenance of synaptic connections. Our previous work showed that 7 month old human apoE4 targeted replacement (TR) mice displayed significant synaptic deficits in the principal neurons of the lateral amygdala, a region that is critical for memory formation and also one of the primary regions affected in Alzheimer's disease, compared to apoE3 TR mice. In the current study, we determined how age and varying APOE genotype affect synaptic integrity of amygdala neurons by comparing electrophysiological and morphometric properties in C57BL6, apoE knockout, and human apoE3, E4 and E2/4 TR mice at 1 month and 7 months. The apoE4 TR mice exhibited the lowest level of excitatory synaptic activity and dendritic arbor compared to other cohorts at both ages, and became progressively worse by 7 months. In contrast, the apoE3 TR mice exhibited the highest synaptic activity and dendritic arbor of all cohorts at both ages. C57BL6 mice displayed virtually identical synaptic activity to apoE3 TR mice at 1 month; however this activity decreased by 7 months. ApoE knockout mice exhibited a similar synaptic activity profile with apoE4 TR mice at 7 months. Consistent with previous reports that APOE2 confers protection, the apoE4-dependent deficits in excitatory activity were significantly attenuated in apoE2/4 TR mice at both ages. These findings demonstrate that expression of human apoE4 contributes to functional deficits in the amygdala very early in development and may be responsible for altering neuronal circuitry that eventually leads to cognitive and affective disorders later in life.
Copyright © 2010 Society for Vascular Surgery. Published by Elsevier Ltd. All rights reserved.
Figures


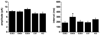
Similar articles
-
Altered neurotransmission in the lateral amygdala in aged human apoE4 targeted replacement mice.Neurobiol Aging. 2014 Sep;35(9):2046-52. doi: 10.1016/j.neurobiolaging.2014.02.019. Epub 2014 Mar 2. Neurobiol Aging. 2014. PMID: 24698766 Free PMC article.
-
Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Aβ metabolism in apoE4-targeted replacement mice.Mol Neurodegener. 2015 Mar 5;10:6. doi: 10.1186/s13024-015-0001-3. Mol Neurodegener. 2015. PMID: 25871773 Free PMC article.
-
ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo.J Neurosci. 2009 Dec 2;29(48):15317-22. doi: 10.1523/JNEUROSCI.4026-09.2009. J Neurosci. 2009. PMID: 19955384 Free PMC article.
-
Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review.
-
A multi-hit hypothesis for an APOE4-dependent pathophysiological state.Eur J Neurosci. 2022 Nov;56(9):5476-5515. doi: 10.1111/ejn.15685. Epub 2022 Jun 6. Eur J Neurosci. 2022. PMID: 35510513 Free PMC article. Review.
Cited by
-
Altered neurotransmission in the lateral amygdala in aged human apoE4 targeted replacement mice.Neurobiol Aging. 2014 Sep;35(9):2046-52. doi: 10.1016/j.neurobiolaging.2014.02.019. Epub 2014 Mar 2. Neurobiol Aging. 2014. PMID: 24698766 Free PMC article.
-
Regional-specific effects of ovarian hormone loss on synaptic plasticity in adult human APOE targeted replacement mice.PLoS One. 2014 Apr 14;9(4):e94071. doi: 10.1371/journal.pone.0094071. eCollection 2014. PLoS One. 2014. PMID: 24732142 Free PMC article.
-
The effects of apolipoprotein E genotype, α-synuclein deficiency, and sex on brain synaptic and Alzheimer's disease-related pathology.Alzheimers Dement (Amst). 2017 Sep 6;10:1-11. doi: 10.1016/j.dadm.2017.08.003. eCollection 2018. Alzheimers Dement (Amst). 2017. PMID: 29159264 Free PMC article.
-
Effect of APOE Genotype on Synaptic Proteins in Earlier Adult Life.J Alzheimers Dis. 2017;59(3):1123-1137. doi: 10.3233/JAD-170316. J Alzheimers Dis. 2017. PMID: 28731446 Free PMC article.
-
ApoE4 induces synaptic and ERG impairments in the retina of young targeted replacement apoE4 mice.PLoS One. 2013 May 31;8(5):e64949. doi: 10.1371/journal.pone.0064949. Print 2013. PLoS One. 2013. PMID: 23741431 Free PMC article.
References
-
- Anderson R, Barnes JC, Bliss TV, Cain DP, Cambon K, Davies HA, Errington ML, Fellows LA, Gray RA, Hoh T, Stewart M, Large CH, Higgins GA. Behavioural, physiological and morphological analysis of a line of apolipoprotein E knockout mouse. Neuroscience. 1998;85:93–110. - PubMed
-
- Basso M, Gelernter J, Yang J, MacAvoy MG, Varma P, Bronen RA, van Dyck CH. Apolipoprotein E epsilon4 is associated with atrophy of the amygdala in Alzheimer's disease. Neurobiol Aging. 2006;27:1416–1424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

