Cellular inhibitor of apoptosis 1 (cIAP-1) degradation by caspase 8 during TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
- PMID: 20951133
- PMCID: PMC2991414
- DOI: 10.1016/j.yexcr.2010.10.005
Cellular inhibitor of apoptosis 1 (cIAP-1) degradation by caspase 8 during TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
Abstract
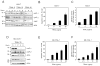
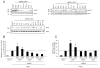
TNF-related apoptosis-inducing ligand (TRAIL) is a potential chemotherapeutic agent with high selectivity for malignant cells. Many tumors, however, are resistant to TRAIL cytotoxicity. Although cellular inhibitors of apoptosis 1 and 2 (cIAP-1 and -2) are often over-expressed in cancers, their role in mediating TRAIL resistance remains unclear. Here, we demonstrate that TRAIL-induced apoptosis of liver cancer cells is associated with degradation of cIAP-1 and X-linked IAP (XIAP), whereas cIAP-2 remains unchanged. Lower concentrations of TRAIL causing minimal or no apoptosis do not alter cIAP-1 or XIAP protein levels. Silencing of cIAP-1 expression, but not XIAP or cIAP-2, as well as co-treatment with a second mitochondrial activator of caspases (SMAC) mimetic (which results in rapid depletion of cIAP-1), sensitizes the cells to TRAIL. TRAIL-induced loss of cIAP-1 and XIAP requires caspase activity. In particular, caspase 8 knockdown stabilizes both cIAP-1 and XIAP, while caspase 9 knockdown prevents XIAP, but not cIAP-1 degradation. Cell-free experiments confirmed cIAP-1 is a substrate for caspase 8, with likely multiple cleavage sites. These results suggest that TRAIL-mediated apoptosis proceeds through caspase 8-dependent degradation of cIAP-1. Targeted depletion of cIAP-1 by SMAC mimetics in conjunction with TRAIL may be beneficial for the treatment of human hepatobiliary malignancies.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



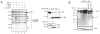
Similar articles
-
A Smac-mimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB.BMC Cancer. 2009 Nov 6;9:392. doi: 10.1186/1471-2407-9-392. BMC Cancer. 2009. PMID: 19895686 Free PMC article.
-
Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis, cell survival and proliferation.Mol Cancer. 2009 Jun 23;8:39. doi: 10.1186/1476-4598-8-39. Mol Cancer. 2009. PMID: 19549337 Free PMC article.
-
Small-molecule IAP antagonists sensitize cancer cells to TRAIL-induced apoptosis: roles of XIAP and cIAPs.Mol Cancer Ther. 2014 Jan;13(1):5-15. doi: 10.1158/1535-7163.MCT-13-0153. Epub 2013 Nov 5. Mol Cancer Ther. 2014. PMID: 24194568 Free PMC article.
-
Using natural products to promote caspase-8-dependent cancer cell death.Cancer Immunol Immunother. 2017 Feb;66(2):223-231. doi: 10.1007/s00262-016-1855-0. Epub 2016 Jun 10. Cancer Immunol Immunother. 2017. PMID: 27286684 Free PMC article. Review.
-
XIAP as a multifaceted molecule in Cellular Signaling.Apoptosis. 2022 Aug;27(7-8):441-453. doi: 10.1007/s10495-022-01734-z. Epub 2022 Jun 4. Apoptosis. 2022. PMID: 35661061 Review.
Cited by
-
Hepatitis C Virus Infection Increases c-Jun N-Terminal Kinase (JNK) Phosphorylation and Accentuates Hepatocyte Lipoapoptosis.Med Sci Monit. 2017 Sep 21;23:4526-4532. doi: 10.12659/msm.903210. Med Sci Monit. 2017. PMID: 28931802 Free PMC article.
-
Intersecting pathways in inflammation and cancer: Hepatocellular carcinoma as a paradigm.World J Clin Oncol. 2012 Feb 10;3(2):15-23. doi: 10.5306/wjco.v3.i2.15. World J Clin Oncol. 2012. PMID: 22347691 Free PMC article.
-
The pyrrolo-1,5-benzoxazepine, PBOX-15, enhances TRAIL‑induced apoptosis by upregulation of DR5 and downregulation of core cell survival proteins in acute lymphoblastic leukaemia cells.Int J Oncol. 2016 Jul;49(1):74-88. doi: 10.3892/ijo.2016.3518. Epub 2016 May 12. Int J Oncol. 2016. PMID: 27176505 Free PMC article.
-
Ablation of the androgen receptor from vascular smooth muscle cells demonstrates a role for testosterone in vascular calcification.Sci Rep. 2016 Apr 20;6:24807. doi: 10.1038/srep24807. Sci Rep. 2016. PMID: 27095121 Free PMC article.
-
Antileukemic Natural Product Induced Both Apoptotic and Pyroptotic Programmed Cell Death and Differentiation Effect.Int J Mol Sci. 2021 Oct 18;22(20):11239. doi: 10.3390/ijms222011239. Int J Mol Sci. 2021. PMID: 34681898 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

