Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKC zeta that modulate neuronal plasticity
- PMID: 20949042
- PMCID: PMC2951910
- DOI: 10.1371/journal.pone.0013191
Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKC zeta that modulate neuronal plasticity
Abstract
Background: Dominant mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common genetic cause of Parkinson's disease, however, the underlying pathogenic mechanisms are poorly understood. Several in vitro studies have shown that the most frequent mutation, LRRK2(G2019S), increases kinase activity and impairs neuronal survival. LRRK2 has been linked to the mitogen-activated protein kinase kinase kinase family and the receptor-interacting protein kinases based on sequence similarity within the kinase domain and in vitro substrate phosphorylation.
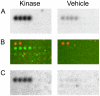
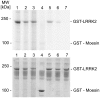
Methodology/principal findings: We used an unbiased proteomic approach to identify the kinase signaling pathways wherein LRRK2 may be active. By incubation of protein microarrays containing 260 signal transduction proteins we detected four arrayed Ste20 serine/threonine kinase family members (TAOK3, STK3, STK24, STK25) as novel LRRK2 substrates and LRRK2 interacting proteins, respectively. Moreover, we found that protein kinase C (PKC) zeta binds and phosphorylates LRRK2 both in vitro and in vivo.
Conclusions/significance: Ste20 kinases and PKC zeta contribute to neuronal Tau phosphorylation, neurite outgrowth and synaptic plasticity under physiological conditions. Our data suggest that these kinases may also be involved in synaptic dysfunction and neurite fragmentation in transgenic mice and in human PD patients carrying toxic gain-of-function LRRK2 mutations.
Conflict of interest statement
Figures





Similar articles
-
14-3-3 Proteins regulate mutant LRRK2 kinase activity and neurite shortening.Hum Mol Genet. 2016 Jan 1;25(1):109-22. doi: 10.1093/hmg/ddv453. Epub 2015 Nov 5. Hum Mol Genet. 2016. PMID: 26546614 Free PMC article.
-
Leucine-rich repeat kinase 2 interacts with p21-activated kinase 6 to control neurite complexity in mammalian brain.J Neurochem. 2015 Dec;135(6):1242-56. doi: 10.1111/jnc.13369. Epub 2015 Oct 19. J Neurochem. 2015. PMID: 26375402 Free PMC article.
-
Phosphoproteomic evaluation of pharmacological inhibition of leucine-rich repeat kinase 2 reveals significant off-target effects of LRRK-2-IN-1.J Neurochem. 2014 Feb;128(4):561-76. doi: 10.1111/jnc.12483. Epub 2013 Nov 11. J Neurochem. 2014. PMID: 24117733
-
Role of LRRK2 kinase activity in the pathogenesis of Parkinson's disease.Biochem Soc Trans. 2012 Oct;40(5):1058-62. doi: 10.1042/BST20120054. Biochem Soc Trans. 2012. PMID: 22988865 Review.
-
Leucine-rich repeat kinase 2 (LRRK2): a key player in the pathogenesis of Parkinson's disease.J Neurosci Res. 2009 May 1;87(6):1283-95. doi: 10.1002/jnr.21949. J Neurosci Res. 2009. PMID: 19025767 Free PMC article. Review.
Cited by
-
LRRK2 mediates axon development by regulating Frizzled3 phosphorylation and growth cone-growth cone communication.Proc Natl Acad Sci U S A. 2020 Jul 28;117(30):18037-18048. doi: 10.1073/pnas.1921878117. Epub 2020 Jul 8. Proc Natl Acad Sci U S A. 2020. PMID: 32641508 Free PMC article.
-
Role of GARP Vesicle Tethering Complex in Golgi Physiology.Int J Mol Sci. 2023 Mar 23;24(7):6069. doi: 10.3390/ijms24076069. Int J Mol Sci. 2023. PMID: 37047041 Free PMC article. Review.
-
Evolution of neurodegeneration.Curr Biol. 2012 Sep 11;22(17):R753-61. doi: 10.1016/j.cub.2012.07.008. Curr Biol. 2012. PMID: 22975006 Free PMC article. Review.
-
Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder.Cells. 2024 Jul 15;13(14):1194. doi: 10.3390/cells13141194. Cells. 2024. PMID: 39056776 Free PMC article.
-
Polygenic effects of common single-nucleotide polymorphisms on life span: when association meets causality.Rejuvenation Res. 2012 Aug;15(4):381-94. doi: 10.1089/rej.2011.1257. Epub 2012 Apr 25. Rejuvenation Res. 2012. PMID: 22533364 Free PMC article.
References
-
- Abeliovich A, Flint BM. Parkinsonism genes: culprits and clues. J Neurochem. 2006;99:1062–1072. - PubMed
-
- Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. - PubMed
-
- Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

