Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma
- PMID: 20948438
- PMCID: PMC2964409
- DOI: 10.1097/CJI.0b013e3181f3cbf4
Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma
Abstract
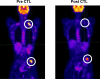
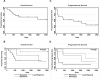
Patients with recurrent or refractory Epstein Barr Virus (EBV)-positive nasopharyngeal carcinoma (NPC) continue to have poor outcomes. Our earlier Phase I dose escalation clinical study of 10 NPC patients showed that infusion of EBV-specific cytotoxic T cells (EBV-CTLs) was safe and had antitumor activity. To better define the overall response rate and discover whether disease status, EBV-antigen specificity, and/or in vivo expansion of infused EBV-CTLs predicted outcome, we treated 13 additional NPC patients with EBV-CTLs in a fixed-dose, Phase II component of the study. We assessed toxicity, efficacy, specificity, and expansion of infused CTLs for all 23 recurrent/refractory NPC patients treated on this Phase I/II clinical study. At the time of CTL infusion, 8 relapsed NPC patients were in remission and 15 had active disease. No significant toxicity was observed. Of the relapsed patients treated in their second or subsequent remission, 62% (5/8) remain disease free (at 17 to 75 mo), whereas 48.7% (7/15) of those with active disease had a CR/CRu (33.3%) or PR (15.4%). In contrast to locoregional disease, metastatic disease was associated with an increased risk of disease progression (HR: 3.91, P=0.015) and decreased overall survival (HR: 5.55, P=0.022). Neither the specificity of the infused CTLs for particular EBV antigens nor their measurable in vivo expansion discernibly influenced outcome. In conclusion, treatment of patients with relapsed/refractory EBV-positive NPC with EBV-CTLs is safe and can be associated with significant, long-term clinical benefit, particularly for patients with locoregional disease.
Figures

Similar articles
-
Epstein-Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma.Cancer. 2017 Jul 15;123(14):2642-2650. doi: 10.1002/cncr.30541. Epub 2017 Feb 21. Cancer. 2017. PMID: 28222215 Clinical Trial.
-
Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma.Ann Oncol. 2004 Jan;15(1):113-7. doi: 10.1093/annonc/mdh027. Ann Oncol. 2004. PMID: 14679129
-
Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes.J Clin Oncol. 2005 Dec 10;23(35):8942-9. doi: 10.1200/JCO.2005.02.6195. Epub 2005 Oct 3. J Clin Oncol. 2005. PMID: 16204009 Clinical Trial.
-
[Value of anti-Epstein-Barr antibody detection in the diagnosis and management of undifferentiated carcinoma of the nasopharynx].Bull Cancer Radiother. 1996;83(1):3-7. Bull Cancer Radiother. 1996. PMID: 8679277 Review. French.
-
Immunotherapy for Epstein-Barr virus-associated cancers in children.Oncologist. 2003;8(1):83-98. doi: 10.1634/theoncologist.8-1-83. Oncologist. 2003. PMID: 12604735 Review.
Cited by
-
Epstein-Barr virus-targeted therapy in nasopharyngeal carcinoma.J Cancer Res Clin Oncol. 2015 Oct;141(10):1845-57. doi: 10.1007/s00432-015-1969-3. Epub 2015 Apr 29. J Cancer Res Clin Oncol. 2015. PMID: 25920375 Clinical Trial.
-
The Epstein-Barr Virus Lytic Protein BZLF1 as a Candidate Target Antigen for Vaccine Development.Cancer Immunol Res. 2015 Jul;3(7):787-94. doi: 10.1158/2326-6066.CIR-14-0242. Epub 2015 Mar 3. Cancer Immunol Res. 2015. PMID: 25735952 Free PMC article.
-
Understanding the interplay between host immunity and Epstein-Barr virus in NPC patients.Emerg Microbes Infect. 2015 Mar;4(3):e20. doi: 10.1038/emi.2015.20. Epub 2015 Mar 25. Emerg Microbes Infect. 2015. PMID: 26038769 Free PMC article. Review.
-
Targeting EBV's Achilles' heel with antigen-specific T cells.Immunotherapy. 2013 Apr;5(4):353-5. doi: 10.2217/imt.13.22. Immunotherapy. 2013. PMID: 23557418 Free PMC article.
-
Phase I study of expanded natural killer cells in combination with cetuximab for recurrent/metastatic nasopharyngeal carcinoma.Cancer Immunol Immunother. 2022 Sep;71(9):2277-2286. doi: 10.1007/s00262-022-03158-9. Epub 2022 Jan 30. Cancer Immunol Immunother. 2022. PMID: 35098345 Free PMC article. Clinical Trial.
References
-
- Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13:1007–15. - PubMed
-
- Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–41. - PubMed
-
- Mould RF, Tai TH. Nasopharyngeal carcinoma: treatments and outcomes in the 20th century. Br J Radiol. 2002;75:307–39. - PubMed
-
- Ayan I, Kaytan E, Ayan N. Childhood nasopharyngeal carcinoma: from biology to treatment. Lancet Oncol. 2003;4:13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

