Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis
- PMID: 20944749
- PMCID: PMC3006164
- DOI: 10.1038/nature09413
Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis
Abstract
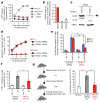
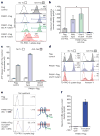
Apoptotic cells release 'find-me' signals at the earliest stages of death to recruit phagocytes. The nucleotides ATP and UTP represent one class of find-me signals, but their mechanism of release is not known. Here, we identify the plasma membrane channel pannexin 1 (PANX1) as a mediator of find-me signal/nucleotide release from apoptotic cells. Pharmacological inhibition and siRNA-mediated knockdown of PANX1 led to decreased nucleotide release and monocyte recruitment by apoptotic cells. Conversely, PANX1 overexpression enhanced nucleotide release from apoptotic cells and phagocyte recruitment. Patch-clamp recordings showed that PANX1 was basally inactive, and that induction of PANX1 currents occurred only during apoptosis. Mechanistically, PANX1 itself was a target of effector caspases (caspases 3 and 7), and a specific caspase-cleavage site within PANX1 was essential for PANX1 function during apoptosis. Expression of truncated PANX1 (at the putative caspase cleavage site) resulted in a constitutively open channel. PANX1 was also important for the 'selective' plasma membrane permeability of early apoptotic cells to specific dyes. Collectively, these data identify PANX1 as a plasma membrane channel mediating the regulated release of find-me signals and selective plasma membrane permeability during apoptosis, and a new mechanism of PANX1 activation by caspases.
Figures


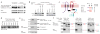
Similar articles
-
Apoptosis: opening PANdora's BoX.Curr Biol. 2010 Nov 9;20(21):R940-2. doi: 10.1016/j.cub.2010.09.066. Curr Biol. 2010. PMID: 21056838
-
A quantized mechanism for activation of pannexin channels.Nat Commun. 2017 Jan 30;8:14324. doi: 10.1038/ncomms14324. Nat Commun. 2017. PMID: 28134257 Free PMC article.
-
Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism.J Biol Chem. 2014 Sep 26;289(39):27246-27263. doi: 10.1074/jbc.M114.590240. Epub 2014 Aug 11. J Biol Chem. 2014. PMID: 25112874 Free PMC article.
-
Revisiting multimodal activation and channel properties of Pannexin 1.J Gen Physiol. 2018 Jan 2;150(1):19-39. doi: 10.1085/jgp.201711888. Epub 2017 Dec 12. J Gen Physiol. 2018. PMID: 29233884 Free PMC article. Review.
-
Intrinsic properties and regulation of Pannexin 1 channel.Channels (Austin). 2014;8(2):103-9. doi: 10.4161/chan.27545. Epub 2014 Jan 13. Channels (Austin). 2014. PMID: 24419036 Free PMC article. Review.
Cited by
-
The Long-Term Pannexin 1 Ablation Produces Structural and Functional Modifications in Hippocampal Neurons.Cells. 2022 Nov 17;11(22):3646. doi: 10.3390/cells11223646. Cells. 2022. PMID: 36429074 Free PMC article.
-
TNFα modulates PANX1 activation to promote ATP release and enhance P2RX7-mediated antitumor immune responses after chemotherapy in colorectal cancer.Cell Death Dis. 2024 Jan 9;15(1):24. doi: 10.1038/s41419-023-06408-5. Cell Death Dis. 2024. PMID: 38195677 Free PMC article.
-
Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells.Am J Physiol Cell Physiol. 2012 Nov 15;303(10):C1034-44. doi: 10.1152/ajpcell.00175.2012. Epub 2012 Sep 12. Am J Physiol Cell Physiol. 2012. PMID: 22972801 Free PMC article.
-
Immunomodulation of wound healing leading to efferocytosis.Smart Med. 2024 Jan 31;3(1):e20230036. doi: 10.1002/SMMD.20230036. eCollection 2024 Feb. Smart Med. 2024. PMID: 39188510 Free PMC article. Review.
-
Clearance of Dying Cells by Phagocytes: Mechanisms and Implications for Disease Pathogenesis.Adv Exp Med Biol. 2016;930:25-49. doi: 10.1007/978-3-319-39406-0_2. Adv Exp Med Biol. 2016. PMID: 27558816 Free PMC article. Review.
References
-
- Lauber K, Blumanthal SC, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. - PubMed
-
- Idziorek T, Estaquier J, De Bels F, Ameisen JC. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods. 1995;185:249–258. - PubMed
-
- Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nature Med. 2009;15:1170–1178. - PubMed
-
- Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

