AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression
- PMID: 20943949
- PMCID: PMC3002392
- DOI: 10.1091/mbc.E10-06-0507
AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression
Abstract
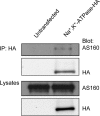
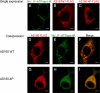
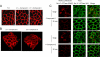
The Na(+),K(+)-ATPase is the major active transport protein found in the plasma membranes of most epithelial cell types. The regulation of Na(+),K(+)-ATPase activity involves a variety of mechanisms, including regulated endocytosis and recycling. Our efforts to identify novel Na(+),K(+)-ATPase binding partners revealed a direct association between the Na(+),K(+)-ATPase and AS160, a Rab-GTPase-activating protein. In COS cells, coexpression of AS160 and Na(+),K(+)-ATPase led to the intracellular retention of the sodium pump. We find that AS160 interacts with the large cytoplasmic NP domain of the α-subunit of the Na(+),K(+)-ATPase. Inhibition of the activity of the adenosine monophosphate-stimulated protein kinase (AMPK) in Madin-Darby canine kidney cells through treatment with Compound C induces Na(+),K(+)-ATPase endocytosis. This effect of Compound C is prevented through the short hairpin RNA-mediated knockdown of AS160, demonstrating that AMPK and AS160 participate in a common pathway to modulate the cell surface expression of the Na(+),K(+)-ATPase.
Figures



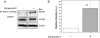
Similar articles
-
Akt Substrate of 160 kD Regulates Na+,K+-ATPase Trafficking in Response to Energy Depletion and Renal Ischemia.J Am Soc Nephrol. 2015 Nov;26(11):2765-76. doi: 10.1681/ASN.2013101040. Epub 2015 Mar 18. J Am Soc Nephrol. 2015. PMID: 25788531 Free PMC article.
-
AMP-activated protein kinase activator A-769662 is an inhibitor of the Na(+)-K(+)-ATPase.Am J Physiol Cell Physiol. 2009 Dec;297(6):C1554-66. doi: 10.1152/ajpcell.00010.2009. Epub 2009 Oct 14. Am J Physiol Cell Physiol. 2009. PMID: 19828836
-
Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta.Mol Cell Biol. 2009 Jul;29(13):3455-64. doi: 10.1128/MCB.00054-09. Epub 2009 Apr 20. Mol Cell Biol. 2009. PMID: 19380482 Free PMC article.
-
Targeting of renal proximal tubule Na,K-ATPase by salt-inducible kinase.Biochem Biophys Res Commun. 2010 Mar 12;393(3):339-44. doi: 10.1016/j.bbrc.2010.02.037. Epub 2010 Feb 10. Biochem Biophys Res Commun. 2010. PMID: 20152810 Free PMC article. Review.
-
Short-term aldosterone action on Na,K-ATPase surface expression: role of aldosterone-induced SGK1?Ann N Y Acad Sci. 2003 Apr;986:554-61. doi: 10.1111/j.1749-6632.2003.tb07253.x. Ann N Y Acad Sci. 2003. PMID: 12763889 Review.
Cited by
-
Activation of AMP-activated protein kinase stimulates Na+,K+-ATPase activity in skeletal muscle cells.J Biol Chem. 2012 Jul 6;287(28):23451-63. doi: 10.1074/jbc.M111.331926. Epub 2012 May 18. J Biol Chem. 2012. PMID: 22610379 Free PMC article.
-
Na(+),K (+)-ATPase as a docking station: protein-protein complexes of the Na(+),K (+)-ATPase.Cell Mol Life Sci. 2013 Jan;70(2):205-22. doi: 10.1007/s00018-012-1039-9. Epub 2012 Jun 14. Cell Mol Life Sci. 2013. PMID: 22695678 Free PMC article. Review.
-
Impairment of Membrane Repolarization Accompanies Axon Transport Deficits in Glaucoma.Front Neurosci. 2019 Nov 1;13:1139. doi: 10.3389/fnins.2019.01139. eCollection 2019. Front Neurosci. 2019. PMID: 31736686 Free PMC article.
-
AMP-Activated Protein Kinase (AMPK)-Dependent Regulation of Renal Transport.Int J Mol Sci. 2018 Nov 6;19(11):3481. doi: 10.3390/ijms19113481. Int J Mol Sci. 2018. PMID: 30404151 Free PMC article. Review.
-
High Throughput Proteomic Exploration of Hypothermic Preservation Reveals Active Processes within the Cell Associated with Cold Ischemia Kinetic.Int J Mol Sci. 2021 Feb 27;22(5):2384. doi: 10.3390/ijms22052384. Int J Mol Sci. 2021. PMID: 33673561 Free PMC article.
References
-
- Al-Khalili L., Kotova O., Tsuchida H., Ehren I., Feraille E., Krook A., Chibalin A. V. ERK1/2 mediates insulin stimulation of Na(+),K(+)-ATPase by phosphorylation of the alpha-subunit in human skeletal muscle cells. J. Biol. Chem. 2004;279:25211–25218. - PubMed
-
- Al-Khalili L., Yu M., Chibalin A. V. Na+,K+-ATPase trafficking in skeletal muscle: insulin stimulates translocation of both alpha 1- and alpha 2-subunit isoforms. FEBS Lett. 2003;536:198–202. - PubMed
-
- Barlet-Bas C., Khadouri C., Marsy S., Doucet A. Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J. Biol. Chem. 1990;265:7799–7803. - PubMed
-
- Bertorello A. M., Komarova Y., Smith K., Leibiger I. B., Efendiev R., Pedemonte C. H., Borisy G., Sznajder J. I. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol. Biol. Cell. 2003;14:1149–1157. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

