Novel drug targets based on metallobiology of Alzheimer's disease
- PMID: 20942746
- PMCID: PMC6677255
- DOI: 10.1517/14728222.2010.525352
Novel drug targets based on metallobiology of Alzheimer's disease
Abstract
Importance of the field: Increased localization of Zn, Fe, Cu and Al within the senile plaques (SP) exacerbates amyloid beta (Aβ)-mediated oxidative damage, and acts as catalyst for Aβ aggregation in Alzheimer's disease (AD). Thus, disruption of aberrant metal-peptide interactions via chelation therapy holds considerable promise as a rational therapeutic strategy against Alzheimer's amyloid pathogenesis.
Areas covered in this review: The complexities of metal-induced genesis of SP are reviewed. The recent advances in the molecular mechanism of action of metal chelating agents are discussed with critical assessment of their potential to become drugs.
What the reader will gain: Taking into consideration the interaction of metals with the metal-responsive elements on the Alzheimer's amyloid precursor protein (APP), readers will gain understanding of several points to bear in mind when developing a screening campaign for AD-therapeutics.
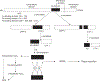
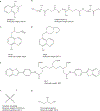
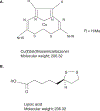
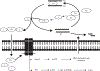
Take home message: A functional iron-responsive element (IRE) RNA stem loop in the 5' untranslated region (UTR) of the APP transcript regulates neural APP translation. Desferrioxamine, clioquinol, tetrathiolmolybdate, dimercaptopropanol, VK-28, and natural antioxidants, such as curcumin and ginko biloba need critical evaluation as AD therapeutics. There is a necessity for novel screens (related to metallobiology) to identify therapeutics effective in AD.
Conflict of interest statement
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Figures


Similar articles
-
Redox-active metals, oxidative stress, and Alzheimer's disease pathology.Ann N Y Acad Sci. 2004 Mar;1012:153-63. doi: 10.1196/annals.1306.012. Ann N Y Acad Sci. 2004. PMID: 15105262 Review.
-
The redox chemistry of the Alzheimer's disease amyloid beta peptide.Biochim Biophys Acta. 2007 Aug;1768(8):1976-90. doi: 10.1016/j.bbamem.2007.02.002. Epub 2007 Feb 9. Biochim Biophys Acta. 2007. PMID: 17433250 Review.
-
An iron-responsive element type II in the 5'-untranslated region of the Alzheimer's amyloid precursor protein transcript.J Biol Chem. 2002 Nov 22;277(47):45518-28. doi: 10.1074/jbc.M207435200. Epub 2002 Aug 26. J Biol Chem. 2002. PMID: 12198135
-
The integrated role of desferrioxamine and phenserine targeted to an iron-responsive element in the APP-mRNA 5'-untranslated region.Ann N Y Acad Sci. 2004 Dec;1035:34-48. doi: 10.1196/annals.1332.003. Ann N Y Acad Sci. 2004. PMID: 15681799
-
Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer's disease: Limitations, and current and future perspectives.J Trace Elem Med Biol. 2021 Sep;67:126779. doi: 10.1016/j.jtemb.2021.126779. Epub 2021 May 15. J Trace Elem Med Biol. 2021. PMID: 34034029 Review.
Cited by
-
Ionic Homeostasis Maintenance in ALS: Focus on New Therapeutic Targets.Front Neurosci. 2018 Aug 7;12:510. doi: 10.3389/fnins.2018.00510. eCollection 2018. Front Neurosci. 2018. PMID: 30131665 Free PMC article. Review.
-
Nitric Oxide, Iron and Neurodegeneration.Front Neurosci. 2019 Feb 18;13:114. doi: 10.3389/fnins.2019.00114. eCollection 2019. Front Neurosci. 2019. PMID: 30833886 Free PMC article. Review.
-
Intranasal deferoxamine attenuates synapse loss via up-regulating the P38/HIF-1α pathway on the brain of APP/PS1 transgenic mice.Front Aging Neurosci. 2015 Jun 2;7:104. doi: 10.3389/fnagi.2015.00104. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26082716 Free PMC article.
-
Towards the prevention of potential aluminum toxic effects and an effective treatment for Alzheimer's disease.J Inorg Biochem. 2011 Nov;105(11):1505-12. doi: 10.1016/j.jinorgbio.2011.08.001. Epub 2011 Aug 19. J Inorg Biochem. 2011. PMID: 22099160 Free PMC article. Review.
-
Treatment with 1, 10 Phenanthroline-5-Amine Reduced Amyloid Burden in a Mouse Model of Alzheimer's Disease.J Alzheimers Dis. 2024;97(1):239-247. doi: 10.3233/JAD-221285. J Alzheimers Dis. 2024. PMID: 38073385 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
