Rapid and transient recruitment of DNMT1 to DNA double-strand breaks is mediated by its interaction with multiple components of the DNA damage response machinery
- PMID: 20940144
- PMCID: PMC3000680
- DOI: 10.1093/hmg/ddq451
Rapid and transient recruitment of DNMT1 to DNA double-strand breaks is mediated by its interaction with multiple components of the DNA damage response machinery
Abstract
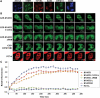
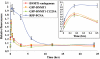
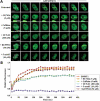
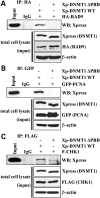
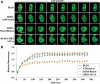
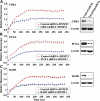
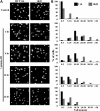
DNA methylation is an epigenetic mark critical for regulating transcription, chromatin structure and genome stability. Although many studies have shed light on how methylation impacts transcription and interfaces with the histone code, far less is known about how it regulates genome stability. We and others have shown that DNA methyltransferase 1 (DNMT1), the maintenance methyltransferase, contributes to the cellular response to DNA damage, yet DNMT1's exact role in this process remains unclear. DNA damage, particularly in the form of double-strand breaks (DSBs), poses a major threat to genome integrity. Cells therefore possess a potent system to respond to and repair DSBs, or to initiate cell death. In the current study, we used a near-infrared laser microirradiation system to directly study the link between DNMT1 and DSBs. Our results demonstrate that DNMT1 is rapidly but transiently recruited to DSBs. DNMT1 recruitment is dependent on its ability to interact with both PCNA and the ATR effector kinase CHK1, but is independent of its catalytic activity. In addition, we show for the first time that DNMT1 interacts with the 9-1-1 PCNA-like sliding clamp and that this interaction also contributes to DNMT1 localization to DNA DSBs. Finally, we demonstrate that DNMT1 modulates the rate of DSB repair and is essential for suppressing abnormal activation of the DNA damage response in the absence of exogenous damage. Taken together, our studies provide compelling additional evidence for DNMT1 acting as a regulator of genome integrity and as an early responder to DNA DSBs.
Figures

Similar articles
-
DNA methylation inhibitor 5-Aza-2'-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B.Mol Cell Biol. 2008 Jan;28(2):752-71. doi: 10.1128/MCB.01799-07. Epub 2007 Nov 8. Mol Cell Biol. 2008. PMID: 17991895 Free PMC article.
-
Replication independent DNA double-strand break retention may prevent genomic instability.Mol Cancer. 2010 Mar 31;9:70. doi: 10.1186/1476-4598-9-70. Mol Cancer. 2010. PMID: 20356374 Free PMC article.
-
PCNA clamp facilitates action of DNA cytosine methyltransferase 1 on hemimethylated DNA.Genes Cells. 2002 Oct;7(10):997-1007. doi: 10.1046/j.1365-2443.2002.00584.x. Genes Cells. 2002. PMID: 12354094
-
DNMT1: catalytic and non-catalytic roles in different biological processes.Epigenomics. 2022 May;14(10):629-643. doi: 10.2217/epi-2022-0035. Epub 2022 Apr 12. Epigenomics. 2022. PMID: 35410490 Review.
-
Maintaining Genome Integrity: Protein Kinases and Phosphatases Orchestrate the Balancing Act of DNA Double-Strand Breaks Repair in Cancer.Int J Mol Sci. 2023 Jun 16;24(12):10212. doi: 10.3390/ijms241210212. Int J Mol Sci. 2023. PMID: 37373360 Free PMC article. Review.
Cited by
-
Changes in one-carbon metabolism and DNA methylation in the hearts of mice exposed to space environment-relevant doses of oxygen ions (16O).Life Sci Space Res (Amst). 2019 Aug;22:8-15. doi: 10.1016/j.lssr.2019.05.003. Epub 2019 May 31. Life Sci Space Res (Amst). 2019. PMID: 31421852 Free PMC article.
-
Mechanisms of DNA Methyltransferase Recruitment in Mammals.Genes (Basel). 2018 Dec 10;9(12):617. doi: 10.3390/genes9120617. Genes (Basel). 2018. PMID: 30544749 Free PMC article. Review.
-
Methylated DNMT1 and E2F1 are targeted for proteolysis by L3MBTL3 and CRL4DCAF5 ubiquitin ligase.Nat Commun. 2018 Apr 24;9(1):1641. doi: 10.1038/s41467-018-04019-9. Nat Commun. 2018. PMID: 29691401 Free PMC article.
-
DNA methyltransferases, DNA damage repair, and cancer.Adv Exp Med Biol. 2013;754:3-29. doi: 10.1007/978-1-4419-9967-2_1. Adv Exp Med Biol. 2013. PMID: 22956494 Free PMC article. Review.
-
Pharmacological inhibition of EZH2 combined with DNA‑damaging agents interferes with the DNA damage response in MM cells.Mol Med Rep. 2019 May;19(5):4249-4255. doi: 10.3892/mmr.2019.10075. Epub 2019 Mar 21. Mol Med Rep. 2019. PMID: 30942459 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

