Capturing the essence of organic synthesis: from bioactive natural products to designed molecules in today's medicine
- PMID: 20936876
- PMCID: PMC2993809
- DOI: 10.1021/jo101606g
Capturing the essence of organic synthesis: from bioactive natural products to designed molecules in today's medicine
Abstract
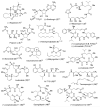
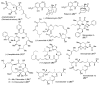
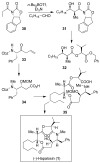
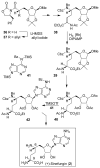
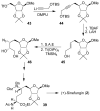
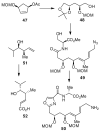
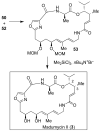
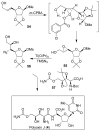
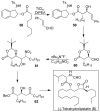
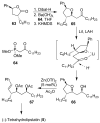
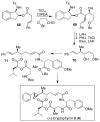
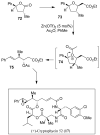
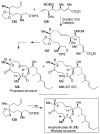
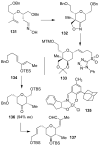
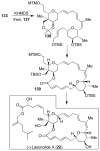
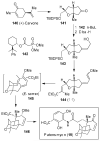
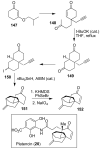
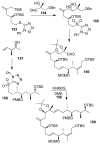
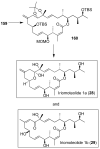
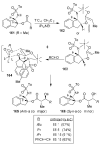
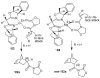
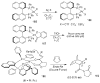
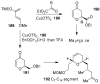
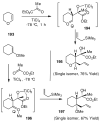
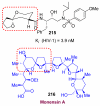
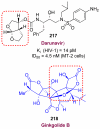
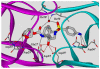
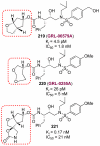
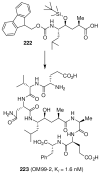
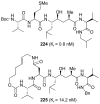
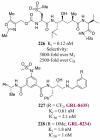
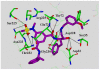
In this Perspective, I outline my group's research involving the chemical syntheses of medicinally important natural products, exploration of their bioactivity, and the development of new asymmetric carbon-carbon bond-forming reactions. This paper also highlights our approach to molecular design and synthesis of conceptually novel inhibitors against target proteins involved in the pathogenesis of human diseases, including AIDS and Alzheimer's disease.
Figures
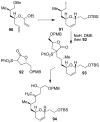
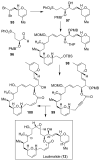
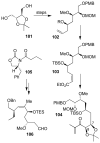
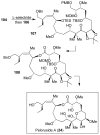
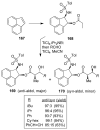
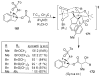

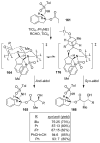
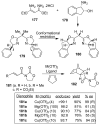
Similar articles
-
Total synthesis of the marine natural product (-)-clavosolide A.Org Lett. 2006 Jul 20;8(15):3319-22. doi: 10.1021/ol0611705. Org Lett. 2006. PMID: 16836395
-
Polyketide construction via hydrohydroxyalkylation and related alcohol C-H functionalizations: reinventing the chemistry of carbonyl addition.Nat Prod Rep. 2014 Apr;31(4):504-13. doi: 10.1039/c3np70076c. Epub 2014 Feb 11. Nat Prod Rep. 2014. PMID: 24514754 Free PMC article. Review.
-
Formal synthesis of palmerolide A, featuring alkynogenic fragmentation and syn-selective vinylogous aldol chemistry.Org Lett. 2013 Feb 15;15(4):886-9. doi: 10.1021/ol400014e. Epub 2013 Feb 1. Org Lett. 2013. PMID: 23373594
-
Controlling the facial selectivity of asymmetric [4+2] cyclo-additions: a concise synthesis of the cis-decalin core structure of superstolides A and B.Org Lett. 2011 Jul 15;13(14):3580-3. doi: 10.1021/ol201095b. Epub 2011 Jun 14. Org Lett. 2011. PMID: 21671636 Free PMC article.
-
Enantioselective Brønsted Acid Catalysis as a Tool for the Synthesis of Natural Products and Pharmaceuticals.Chemistry. 2018 Mar 15;24(16):3925-3943. doi: 10.1002/chem.201703556. Epub 2017 Dec 4. Chemistry. 2018. PMID: 28981209 Review.
Cited by
-
Design of novel HIV-1 protease inhibitors incorporating isophthalamide-derived P2-P3 ligands: Synthesis, biological evaluation and X-ray structural studies of inhibitor-HIV-1 protease complex.Bioorg Med Chem. 2017 Oct 1;25(19):5114-5127. doi: 10.1016/j.bmc.2017.04.005. Epub 2017 Apr 9. Bioorg Med Chem. 2017. PMID: 28434781 Free PMC article.
-
Beyond darunavir: recent development of next generation HIV-1 protease inhibitors to combat drug resistance.Chem Commun (Camb). 2022 Oct 20;58(84):11762-11782. doi: 10.1039/d2cc04541a. Chem Commun (Camb). 2022. PMID: 36200462 Free PMC article. Review.
-
Novel 2-Aryloxazoline Compounds Exhibit an Inhibitory Effect on Candida spp., Including Antifungal-Resistant Isolates.ACS Med Chem Lett. 2020 Nov 23;11(12):2470-2475. doi: 10.1021/acsmedchemlett.0c00449. eCollection 2020 Dec 10. ACS Med Chem Lett. 2020. PMID: 33335669 Free PMC article.
-
Cu(II)-catalyzed olefin migration and Prins cyclization: highly diastereoselective synthesis of substituted tetrahydropyrans.Org Lett. 2011 Aug 19;13(16):4328-31. doi: 10.1021/ol2016675. Epub 2011 Jul 28. Org Lett. 2011. PMID: 21797234 Free PMC article.
-
Nature Inspired Molecular Design: Stereoselective Synthesis of Bicyclic and Polycyclic Ethers for Potent HIV-1 Protease Inhibitors.Asian J Org Chem. 2018 Aug;7(8):1448-1466. doi: 10.1002/ajoc.201800255. Epub 2018 Jun 8. Asian J Org Chem. 2018. PMID: 31595212 Free PMC article.
References
-
-
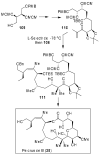
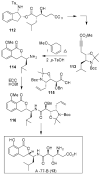
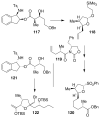
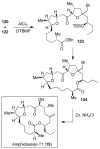
Fenical W, Jensen PR. Nature Chem. Biol. 2006;2:666–673. Wilson RM, Danishefsky SJ. J. Org. Chem. 2006;71:8329–8351. Paterson I, Anderson EA. Science. 2005;310:451–453. Clardy J, Walsh C. Nature. 2004;432:829–837. and references cited therein.
-
-
- Haustedt LO, Mang C, Siems K, Schiewe H. Curr. Opin. Drug Discov. Devel. 2006;9:445–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

