Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate
- PMID: 20926660
- PMCID: PMC3001133
- DOI: 10.1523/JNEUROSCI.2108-10.2010
Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate
Abstract
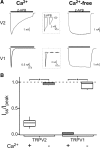
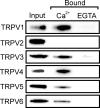
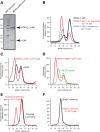
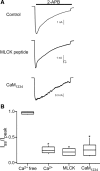
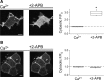
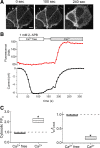
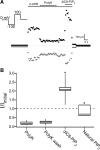
TRPV2 is a member of the transient receptor potential family of ion channels involved in chemical and thermal pain transduction. Unlike the related TRPV1 channel, TRPV2 does not appear to bind either calmodulin or ATP in its N-terminal ankyrin repeat domain. In addition, it does not contain a calmodulin-binding site in the distal C-terminal region, as has been proposed for TRPV1. We have found that TRPV2 channels transiently expressed in F-11 cells undergo Ca(2+)-dependent desensitization, similar to the other TRPVs, suggesting that the mechanism of desensitization may be conserved in the subfamily of TRPV channels. TRPV2 desensitization was not altered in whole-cell recordings in the presence of calmodulin inhibitors or on coexpression of mutant calmodulin but was sensitive to changes in membrane phosphatidylinositol 4,5-bisphosphate (PIP(2)), suggesting a role of membrane PIP(2) in TRPV2 desensitization. Simultaneous confocal imaging and electrophysiological recording of cells expressing TRPV2 and a fluorescent PIP(2)-binding probe demonstrated that TRPV2 desensitization was concomitant with depletion of PIP(2). We conclude that the decrease in PIP(2) levels on channel activation underlies a major component of Ca(2+)-dependent desensitization of TRPV2 and may play a similar role in other TRP channels.
Figures
Similar articles
-
Dual regulation of TRPV1 by phosphoinositides.J Neurosci. 2007 Jun 27;27(26):7070-80. doi: 10.1523/JNEUROSCI.1866-07.2007. J Neurosci. 2007. PMID: 17596456 Free PMC article.
-
Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization.J Physiol. 2007 Aug 15;583(Pt 1):175-93. doi: 10.1113/jphysiol.2007.133231. Epub 2007 Jun 21. J Physiol. 2007. PMID: 17584831 Free PMC article.
-
PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain.Nat Neurosci. 2005 May;8(5):626-34. doi: 10.1038/nn1451. Epub 2005 Apr 24. Nat Neurosci. 2005. PMID: 15852009
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
TRPV2.Handb Exp Pharmacol. 2014;222:247-72. doi: 10.1007/978-3-642-54215-2_10. Handb Exp Pharmacol. 2014. PMID: 24756709 Review.
Cited by
-
Phosphoinositide signaling in somatosensory neurons.Adv Biol Regul. 2016 May;61:2-16. doi: 10.1016/j.jbior.2015.11.012. Epub 2015 Dec 19. Adv Biol Regul. 2016. PMID: 26724974 Free PMC article. Review.
-
What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel?FEBS J. 2013 Nov;280(21):5471-87. doi: 10.1111/febs.12302. Epub 2013 May 28. FEBS J. 2013. PMID: 23615321 Free PMC article. Review.
-
Novel inhibitor candidates of TRPV2 prevent damage of dystrophic myocytes and ameliorate against dilated cardiomyopathy in a hamster model.Oncotarget. 2018 Feb 8;9(18):14042-14057. doi: 10.18632/oncotarget.24449. eCollection 2018 Mar 6. Oncotarget. 2018. PMID: 29581825 Free PMC article.
-
Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis.J Gen Physiol. 2011 Mar;137(3):271-88. doi: 10.1085/jgp.200910388. Epub 2011 Feb 14. J Gen Physiol. 2011. PMID: 21321070 Free PMC article.
-
Phosphoinositides regulate ion channels.Biochim Biophys Acta. 2015 Jun;1851(6):844-56. doi: 10.1016/j.bbalip.2014.09.010. Epub 2014 Sep 18. Biochim Biophys Acta. 2015. PMID: 25241941 Free PMC article. Review.
References
-
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. - PubMed
-
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY010329/EY/NEI NIH HHS/United States
- R01 EY017564/EY/NEI NIH HHS/United States
- P30 EY001730/EY/NEI NIH HHS/United States
- 5T32EY007031-33/EY/NEI NIH HHS/United States
- S10 RR025429/RR/NCRR NIH HHS/United States
- R01EY017564/EY/NEI NIH HHS/United States
- R01 EY017564-04S1/EY/NEI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30EY01730/EY/NEI NIH HHS/United States
- 2R01EY010329-16/EY/NEI NIH HHS/United States
- S10 RR025429-01/RR/NCRR NIH HHS/United States
- R01 EY017564-05/EY/NEI NIH HHS/United States
- T32 EY007031/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
