Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons
- PMID: 20920792
- PMCID: PMC2953270
- DOI: 10.1016/j.neuron.2010.09.022
Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons
Abstract
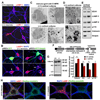
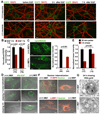
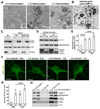
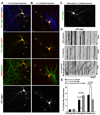
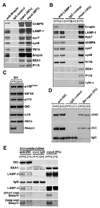
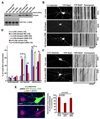
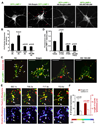
Neuron maintenance and survival require late endocytic transport from distal processes to the soma where lysosomes are predominantly localized. Here, we report a role for Snapin in attaching dynein to late endosomes through its intermediate chain (DIC). snapin(-/-) neurons exhibit aberrant accumulation of immature lysosomes, clustering and impaired retrograde transport of late endosomes along processes, reduced lysosomal proteolysis due to impaired delivery of internalized proteins and hydrolase precursors from late endosomes to lysosomes, and impaired clearance of autolysosomes, combined with reduced neuron viability and neurodegeneration. The phenotypes are rescued by expressing the snapin transgene, but not the DIC-binding-defective Snapin-L99K mutant. Snapin overexpression in wild-type neurons enhances late endocytic transport and lysosomal function, whereas expressing the mutant defective in Snapin-DIC coupling shows a dominant-negative effect. Altogether, our study highlights new mechanistic insights into how Snapin-DIC coordinates retrograde transport and late endosomal-lysosomal trafficking critical for autophagy-lysosomal function, and thus neuronal homeostasis.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Snapin snaps into the dynein complex for late endosome-lysosome trafficking and autophagy.Neuron. 2010 Oct 6;68(1):4-6. doi: 10.1016/j.neuron.2010.09.036. Neuron. 2010. PMID: 20920785
Similar articles
-
Snapin deficiency is associated with developmental defects of the central nervous system.Biosci Rep. 2011 Apr;31(2):151-8. doi: 10.1042/BSR20100110. Biosci Rep. 2011. PMID: 20946101 Free PMC article.
-
Uncovering the role of Snapin in regulating autophagy-lysosomal function.Autophagy. 2011 Apr;7(4):445-7. doi: 10.4161/auto.7.4.14682. Epub 2011 Apr 1. Autophagy. 2011. PMID: 21233602 Free PMC article.
-
Snapin-mediated BACE1 retrograde transport is essential for its degradation in lysosomes and regulation of APP processing in neurons.Cell Rep. 2014 Jan 16;6(1):24-31. doi: 10.1016/j.celrep.2013.12.008. Epub 2013 Dec 27. Cell Rep. 2014. PMID: 24373968 Free PMC article.
-
Defective retrograde transport impairs autophagic clearance in Alzheimer disease neurons.Autophagy. 2017 May 4;13(5):982-984. doi: 10.1080/15548627.2017.1291114. Epub 2017 Feb 28. Autophagy. 2017. PMID: 28318364 Free PMC article. Review.
-
Roles of ESCRT in autophagy-associated neurodegeneration.Autophagy. 2008 Feb;4(2):230-2. doi: 10.4161/auto.5384. Epub 2007 Dec 6. Autophagy. 2008. PMID: 18094607 Review.
Cited by
-
Do Changes in Synaptic Autophagy Underlie the Cognitive Impairments in Huntington's Disease?J Huntingtons Dis. 2021;10(2):227-238. doi: 10.3233/JHD-200466. J Huntingtons Dis. 2021. PMID: 33780373 Free PMC article. Review.
-
Snapin is critical for presynaptic homeostatic plasticity.J Neurosci. 2012 Jun 20;32(25):8716-24. doi: 10.1523/JNEUROSCI.5465-11.2012. J Neurosci. 2012. PMID: 22723711 Free PMC article.
-
Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes.J Cell Biol. 2015 May 11;209(3):377-86. doi: 10.1083/jcb.201412046. Epub 2015 May 4. J Cell Biol. 2015. PMID: 25940348 Free PMC article.
-
JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes.PLoS Genet. 2013;9(2):e1003303. doi: 10.1371/journal.pgen.1003303. Epub 2013 Feb 28. PLoS Genet. 2013. PMID: 23468645 Free PMC article.
-
Snapin deficiency is associated with developmental defects of the central nervous system.Biosci Rep. 2011 Apr;31(2):151-8. doi: 10.1042/BSR20100110. Biosci Rep. 2011. PMID: 20946101 Free PMC article.
References
-
- Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J. Cell Sci. 1997;110:2027–2040. - PubMed
-
- Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr. Biol. 2005;15:360–365. - PubMed
-
- Chen CS, Chen WN, Zhou M, Arttamangkul S, Haugland RP. Probing the cathepsin D using a BODIPY FL-pepstatin A: applications in fluorescence polarization and microscopy. J Biochem. Biophys. Methods. 2000;42:137–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

