p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both
- PMID: 20890124
- PMCID: PMC3359490
- DOI: 10.4161/auto.6.8.13426
p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both
Abstract
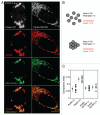
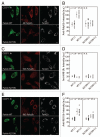
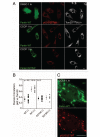
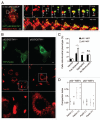
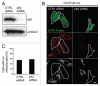
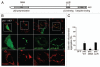
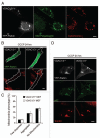
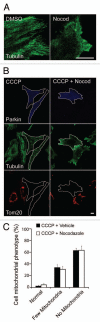
Mitochondria sustain damage with aging, and the resulting mitochondrial dysfunction has been implicated in a number of diseases including Parkinson disease. We recently demonstrated that the E3 ubiquitin ligase Parkin, which is linked to recessive forms of parkinsonism, causes a dramatic increase in mitophagy and a change in mitochondrial distribution, following its translocation from the cytosol to mitochondria. Investigating how Parkin induces these changes may offer insight into the mechanisms that lead to the sequestration and elimination of damaged mitochondria. We report that following Parkin’s translocation from the cytosol to mitochondria, Parkin (but not a pathogenic mutant) promotes the K63-linked polyubiquitination of mitochondrial substrate(s) and recruits the ubiquitin- and LC3-binding protein, p62/SQSTM1, to mitochondria. After its recruitment, p62/SQSTM1 mediates the aggregation of dysfunctional mitochondria through polymerization via its PB1 domain, in a manner analogous to its aggregation of polyubiquitinated proteins. Surprisingly and in contrast to what has been recently reported for ubiquitin-induced pexophagy and xenophagy, p62 appears to be dispensable for mitophagy. Similarly, mitochondrial-anchored ubiquitin is sufficient to recruit p62 and promote mitochondrial clustering, but does not promote mitophagy. Although VDAC1 (but not VDAC2) is ubiquitinated following mitochondrial depolarization, we find VDAC1 cannot fully account for the mitochondrial K63-linked ubiquitin immunoreactivity observed following depolarization, as it is also observed in VDAC1/3-/- mouse embryonic fibroblasts. Additionally, we find VDAC1 and VDAC3 are dispensable for the recruitment of p62, mitochondrial clustering and mitophagy. These results demonstrate that mitochondria are aggregated by p62, following its recruitment by Parkin in a VDAC1-independent manner. They also suggest that proteins other than p62 are likely required for mitophagy downstream of Parkin substrates other than VDAC1.
Figures


Similar articles
-
Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation.Proc Natl Acad Sci U S A. 2014 Oct 21;111(42):E4439-48. doi: 10.1073/pnas.1405752111. Epub 2014 Oct 7. Proc Natl Acad Sci U S A. 2014. PMID: 25294927 Free PMC article.
-
PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1.Nat Cell Biol. 2010 Feb;12(2):119-31. doi: 10.1038/ncb2012. Epub 2010 Jan 24. Nat Cell Biol. 2010. PMID: 20098416
-
Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1.PLoS One. 2011;6(6):e20975. doi: 10.1371/journal.pone.0020975. Epub 2011 Jun 8. PLoS One. 2011. PMID: 21687634 Free PMC article.
-
Mechanisms of PINK1, ubiquitin and Parkin interactions in mitochondrial quality control and beyond.Cell Mol Life Sci. 2019 Dec;76(23):4589-4611. doi: 10.1007/s00018-019-03203-4. Epub 2019 Jun 28. Cell Mol Life Sci. 2019. PMID: 31254044 Free PMC article. Review.
-
Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control.Antioxid Redox Signal. 2011 May 15;14(10):1929-38. doi: 10.1089/ars.2010.3799. Epub 2011 Mar 3. Antioxid Redox Signal. 2011. PMID: 21194381 Free PMC article. Review.
Cited by
-
Role of mitophagy in the neurodegenerative diseases and its pharmacological advances: A review.Front Mol Neurosci. 2022 Oct 4;15:1014251. doi: 10.3389/fnmol.2022.1014251. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36267702 Free PMC article. Review.
-
Therapeutic targeting of autophagy: potential and concerns in treating cardiovascular disease.Circ Res. 2015 Jan 30;116(3):489-503. doi: 10.1161/CIRCRESAHA.116.303791. Circ Res. 2015. PMID: 25634972 Free PMC article. Review.
-
Role of p62/SQSTM1 in liver physiology and pathogenesis.Exp Biol Med (Maywood). 2013 May;238(5):525-38. doi: 10.1177/1535370213489446. Exp Biol Med (Maywood). 2013. PMID: 23856904 Free PMC article. Review.
-
Receptor protein complexes are in control of autophagy.Autophagy. 2012 Nov;8(11):1701-5. doi: 10.4161/auto.21332. Epub 2012 Aug 9. Autophagy. 2012. PMID: 22874568 Free PMC article.
-
Parkinson's disease and autophagy.Parkinsons Dis. 2012;2012:429524. doi: 10.1155/2012/429524. Epub 2012 Oct 17. Parkinsons Dis. 2012. PMID: 23125941 Free PMC article.
References
-
- Orr WC, Sohal RS. Extension of life-span by over-expression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
