Diffusion imaging of brain tumors
- PMID: 20886568
- PMCID: PMC3000221
- DOI: 10.1002/nbm.1544
Diffusion imaging of brain tumors
Abstract
MRI offers a tremendous armamentarium of different methods that can be employed in brain tumor characterization. MR diffusion imaging has become a widely accepted method to probe for the presence of fluid pools and molecular tissue water mobility. For most clinical applications of diffusion imaging, it is assumed that the diffusion signal vs diffusion weighting factor b decays monoexponentially. Within this framework, the measurement of a single diffusion coefficient in brain tumors permits an approximate categorization of tumor type and, for some tumors, definitive diagnosis. In most brain tumors, when compared with normal brain tissue, the diffusion coefficient is elevated. The presence of peritumoral edema, which also exhibits an elevated diffusion coefficient, often precludes the delineation of the tumor on the basis of diffusion information alone. Serially obtained diffusion data are useful to document and even predict the cellular response to drug or radiation therapy. Diffusion measurements in tissues over an extended range of b factors have clearly shown that the monoparametric description of the MR diffusion signal decay is incomplete. Very high diffusion weighting on clinical systems requires substantial compromise in spatial resolution. However, after suitable analysis, superior separation of malignant brain tumors, peritumoral edema and normal brain tissue can be achieved. These findings are also discussed in the light of tissue-specific differences in membrane structure and the restrictions exerted by membranes on diffusion. Finally, measurement of the directional dependence of diffusion permits the assessment of white matter integrity and dislocation. Such information, particularly in conjunction with advanced post-processing, is considered to be immensely useful for therapy planning. Diffusion imaging, which permits monoexponential analysis and provides directional diffusion information, is performed routinely in brain tumor patients. More advanced methods require improvement in acquisition speed and spatial resolution to gain clinical acceptance.
Copyright © 2010 John Wiley & Sons, Ltd.
Figures
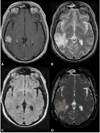
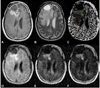


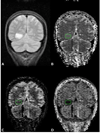
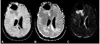

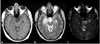
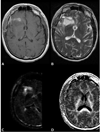
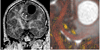
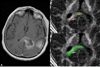
Similar articles
-
Characterization of normal brain and brain tumor pathology by chisquares parameter maps of diffusion-weighted image data.Eur J Radiol. 2003 Mar;45(3):199-207. doi: 10.1016/s0720-048x(02)00308-x. Eur J Radiol. 2003. PMID: 12595104
-
Measurements of diagnostic examination performance using quantitative apparent diffusion coefficient and proton MR spectroscopic imaging in the preoperative evaluation of tumor grade in cerebral gliomas.Eur J Radiol. 2011 Nov;80(2):462-70. doi: 10.1016/j.ejrad.2010.07.017. Epub 2010 Aug 13. Eur J Radiol. 2011. PMID: 20708868
-
Diffusion-weighted MR of the brain: methodology and clinical application.Radiol Med. 2005 Mar;109(3):155-97. Radiol Med. 2005. PMID: 15775887 Review. English, Italian.
-
Diffusion MR imaging: an important tool in the assessment of brain tumors.Neuroimaging Clin N Am. 2011 Feb;21(1):27-49, vii. doi: 10.1016/j.nic.2011.01.010. Neuroimaging Clin N Am. 2011. PMID: 21477750
-
Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity.Radiology. 2007 Nov;245(2):367-84. doi: 10.1148/radiol.2452060445. Radiology. 2007. PMID: 17940300 Review.
Cited by
-
Diffusion tensor magnetic resonance imaging of rat glioma models: a correlation study of MR imaging and histology.J Comput Assist Tomogr. 2012 Nov-Dec;36(6):739-44. doi: 10.1097/RCT.0b013e3182685436. J Comput Assist Tomogr. 2012. PMID: 23192213 Free PMC article.
-
Demonstration of Non-Gaussian Restricted Diffusion in Tumor Cells Using Diffusion Time-Dependent Diffusion-Weighted Magnetic Resonance Imaging Contrast.Front Oncol. 2016 Aug 2;6:179. doi: 10.3389/fonc.2016.00179. eCollection 2016. Front Oncol. 2016. PMID: 27532028 Free PMC article.
-
[Diffusion-Weighted Magnetic Resonance Imaging of Spine].Taehan Yongsang Uihakhoe Chi. 2020 Jan;81(1):58-69. doi: 10.3348/jksr.2020.81.1.58. Epub 2020 Jan 31. Taehan Yongsang Uihakhoe Chi. 2020. PMID: 36238128 Free PMC article. Review. Korean.
-
Correlation Between Apparent Diffusion Coefficient and the Ki-67 Proliferation Index in Grading Pediatric Glioma.J Comput Assist Tomogr. 2023 Mar-Apr 01;47(2):322-328. doi: 10.1097/RCT.0000000000001400. J Comput Assist Tomogr. 2023. PMID: 36957971 Free PMC article.
-
Quantitative parameters of diffusion tensor imaging in the evaluation of carpal tunnel syndrome.Quant Imaging Med Surg. 2022 Jun;12(6):3379-3390. doi: 10.21037/qims-21-910. Quant Imaging Med Surg. 2022. PMID: 35655836 Free PMC article.
References
-
- Essig M, Hawighorst H, Schoenberg SO, Engenhart-Cabillic R, Fuss M, Debus J, Zuna I, Knopp MV, van Kaick G. Fast fluid-attenuated inversion-recovery (FLAIR) MRI in the assessment of intraaxial brain tumors. J Magn Reson Imaging. 1998;8(4):789–798. - PubMed
-
- Cha S. Perfusion MR imaging of brain tumors. Top Magn Reson Imaging. 2004;15(5):279–289. Review. - PubMed
-
- Sehgal V, Delproposto Z, Haddar D, Haacke EM, Sloan AE, Zamorano LJ, Barger G, Hu J, Xu Y, Prabhakaran KP, Elangovan IR, Neelavalli J, Reichenbach JR. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging. 2006;24(1):41–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

