Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner
- PMID: 20886108
- PMCID: PMC2944813
- DOI: 10.1371/journal.ppat.1001121
Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner
Abstract
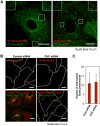
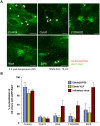
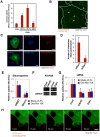
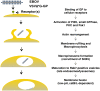
Ebolavirus (EBOV) is an enveloped, single-stranded, negative-sense RNA virus that causes severe hemorrhagic fever with mortality rates of up to 90% in humans and nonhuman primates. Previous studies suggest roles for clathrin- or caveolae-mediated endocytosis in EBOV entry; however, ebolavirus virions are long, filamentous particles that are larger than the plasma membrane invaginations that characterize clathrin- or caveolae-mediated endocytosis. The mechanism of EBOV entry remains, therefore, poorly understood. To better understand Ebolavirus entry, we carried out internalization studies with fluorescently labeled, biologically contained Ebolavirus and Ebolavirus-like particles (Ebola VLPs), both of which resemble authentic Ebolavirus in their morphology. We examined the mechanism of Ebolavirus internalization by real-time analysis of these fluorescently labeled Ebolavirus particles and found that their internalization was independent of clathrin- or caveolae-mediated endocytosis, but that they co-localized with sorting nexin (SNX) 5, a marker of macropinocytosis-specific endosomes (macropinosomes). Moreover, the internalization of Ebolavirus virions accelerated the uptake of a macropinocytosis-specific cargo, was associated with plasma membrane ruffling, and was dependent on cellular GTPases and kinases involved in macropinocytosis. A pseudotyped vesicular stomatitis virus possessing the Ebolavirus glycoprotein (GP) also co-localized with SNX5 and its internalization and infectivity were affected by macropinocytosis inhibitors. Taken together, our data suggest that Ebolavirus is internalized into cells by stimulating macropinocytosis in a GP-dependent manner. These findings provide new insights into the lifecycle of Ebolavirus and may aid in the development of therapeutics for Ebolavirus infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes.PLoS Pathog. 2010 Sep 16;6(9):e1001110. doi: 10.1371/journal.ppat.1001110. PLoS Pathog. 2010. PMID: 20862315 Free PMC article.
-
Ebola virus triggers receptor tyrosine kinase-dependent signaling to promote the delivery of viral particles to entry-conducive intracellular compartments.PLoS Pathog. 2021 Jan 29;17(1):e1009275. doi: 10.1371/journal.ppat.1009275. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33513206 Free PMC article.
-
Ebola virus uses clathrin-mediated endocytosis as an entry pathway.Virology. 2010 May 25;401(1):18-28. doi: 10.1016/j.virol.2010.02.015. Epub 2010 Mar 3. Virology. 2010. PMID: 20202662 Free PMC article.
-
Molecular Mechanism of Externalization of Phosphatidylserine on the Surface of Ebola Virus Particles.DNA Cell Biol. 2019 Feb;38(2):115-120. doi: 10.1089/dna.2018.4485. Epub 2019 Jan 7. DNA Cell Biol. 2019. PMID: 30615471 Review.
-
Filovirus entry: a novelty in the viral fusion world.Viruses. 2012 Feb;4(2):258-75. doi: 10.3390/v4020258. Epub 2012 Feb 7. Viruses. 2012. PMID: 22470835 Free PMC article. Review.
Cited by
-
siRNA Screen Identifies Trafficking Host Factors that Modulate Alphavirus Infection.PLoS Pathog. 2016 Mar 31;12(3):e1005466. doi: 10.1371/journal.ppat.1005466. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 27031835 Free PMC article.
-
Importance of Endocytosis for the Biological Activity of Cedar Virus Fusion Protein.Cells. 2020 Sep 8;9(9):2054. doi: 10.3390/cells9092054. Cells. 2020. PMID: 32911832 Free PMC article.
-
Ebola virus entry: a curious and complex series of events.PLoS Pathog. 2015 Apr 30;11(4):e1004731. doi: 10.1371/journal.ppat.1004731. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25928849 Free PMC article. Review. No abstract available.
-
Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review.
-
Ebola virus exploits a monocyte differentiation program to promote its entry.J Virol. 2013 Apr;87(7):3801-14. doi: 10.1128/JVI.02695-12. Epub 2013 Jan 23. J Virol. 2013. PMID: 23345511 Free PMC article.
References
-
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–1545. - PubMed
-
- Marsh M, Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980;142:439–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

