Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway
- PMID: 20876311
- PMCID: PMC2964568
- DOI: 10.1084/jem.20101074
Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway
Abstract
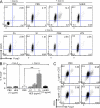
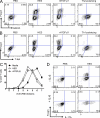
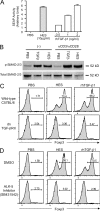
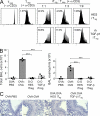
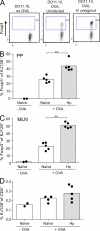
Foxp3-expressing regulatory T (T reg) cells have been implicated in parasite-driven inhibition of host immunity during chronic infection. We addressed whether parasites can directly induce T reg cells. Foxp3 expression was stimulated in naive Foxp3⁻ T cells in mice infected with the intestinal helminth Heligmosomoides polygyrus. In vitro, parasite-secreted proteins (termed H. polygyrus excretory-secretory antigen [HES]) induced de novo Foxp3 expression in fluorescence-sorted Foxp3⁻ splenocytes from Foxp3-green fluorescent protein reporter mice. HES-induced T reg cells suppressed both in vitro effector cell proliferation and in vivo allergic airway inflammation. HES ligated the transforming growth factor (TGF) β receptor and promoted Smad2/3 phosphorylation. Foxp3 induction by HES was lost in dominant-negative TGF-βRII cells and was abolished by the TGF-β signaling inhibitor SB431542. This inhibitor also reduced worm burdens in H. polygyrus-infected mice. HES induced IL-17 in the presence of IL-6 but did not promote Th1 or Th2 development under any conditions. Importantly, antibody to mammalian TGF-β did not recognize HES, whereas antisera that inhibited HES did not affect TGF-β. Foxp3 was also induced by secreted products of Teladorsagia circumcincta, a related nematode which is widespread in ruminant animals. We have therefore identified a novel pathway through which helminth parasites may stimulate T reg cells, which is likely to be a key part of the parasite's immunological relationship with the host.
Figures

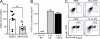
Similar articles
-
Cutting edge: in the absence of TGF-β signaling in T cells, fewer CD103+ regulatory T cells develop, but exuberant IFN-γ production renders mice more susceptible to helminth infection.J Immunol. 2012 Aug 1;189(3):1113-7. doi: 10.4049/jimmunol.1200991. Epub 2012 Jun 29. J Immunol. 2012. PMID: 22753928 Free PMC article.
-
Role of T cell TGF-beta signaling in intestinal cytokine responses and helminthic immune modulation.Eur J Immunol. 2009 Jul;39(7):1870-8. doi: 10.1002/eji.200838956. Eur J Immunol. 2009. PMID: 19544487 Free PMC article.
-
Heligmosomoides polygyrus bakeri Infection Decreases Smad7 Expression in Intestinal CD4+ T Cells, Which Allows TGF-β to Induce IL-10-Producing Regulatory T Cells That Block Colitis.J Immunol. 2019 Apr 15;202(8):2473-2481. doi: 10.4049/jimmunol.1801392. Epub 2019 Mar 8. J Immunol. 2019. PMID: 30850474 Free PMC article.
-
Immune modulation and modulators in Heligmosomoides polygyrus infection.Exp Parasitol. 2012 Sep;132(1):76-89. doi: 10.1016/j.exppara.2011.08.011. Epub 2011 Aug 22. Exp Parasitol. 2012. PMID: 21875581 Free PMC article. Review.
-
Distinct regulatory CD4+T cell subsets; differences between naïve and antigen specific T regulatory cells.Curr Opin Immunol. 2011 Oct;23(5):641-7. doi: 10.1016/j.coi.2011.07.012. Epub 2011 Aug 11. Curr Opin Immunol. 2011. PMID: 21840184 Review.
Cited by
-
miRNA signature of mouse helper T cell hyper-proliferation.PLoS One. 2013 Jun 25;8(6):e66709. doi: 10.1371/journal.pone.0066709. Print 2013. PLoS One. 2013. PMID: 23825558 Free PMC article.
-
Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease.Am J Reprod Immunol. 2013 Apr;69(4):346-58. doi: 10.1111/aji.12083. Epub 2013 Feb 25. Am J Reprod Immunol. 2013. PMID: 23432802 Free PMC article. Review.
-
Trichinella spiralis -induced immunomodulation signatures on gut microbiota and metabolic pathways in mice.PLoS Pathog. 2024 Jan 2;20(1):e1011893. doi: 10.1371/journal.ppat.1011893. eCollection 2024 Jan. PLoS Pathog. 2024. PMID: 38166140 Free PMC article.
-
Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence.Pathogens. 2024 Jul 23;13(8):608. doi: 10.3390/pathogens13080608. Pathogens. 2024. PMID: 39204209 Free PMC article. Review.
-
Helminth infections and host immune regulation.Clin Microbiol Rev. 2012 Oct;25(4):585-608. doi: 10.1128/CMR.05040-11. Clin Microbiol Rev. 2012. PMID: 23034321 Free PMC article. Review.
References
-
- Akdis M., Verhagen J., Taylor A., Karamloo F., Karagiannidis C., Crameri R., Thunberg S., Deniz G., Valenta R., Fiebig H., et al. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199:1567–1575 10.1084/jem.20032058 - DOI - PMC - PubMed
-
- Babu S., Blauvelt C.P., Kumaraswami V., Nutman T.B. 2006. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 176:3248–3256 - PubMed
-
- Baumgart M., Tompkins F., Leng J., Hesse M. 2006. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 176:5374–5387 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

