HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment
- PMID: 20876310
- PMCID: PMC2964584
- DOI: 10.1084/jem.20100587
HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment
Abstract
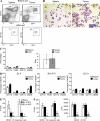
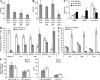
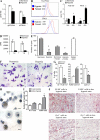
Myeloid-derived suppressor cells (MDSCs) are a major component of the immune-suppressive network described in cancer and many other pathological conditions. We demonstrate that although MDSCs from peripheral lymphoid organs and the tumor site share similar phenotype and morphology, these cells display profound functional differences. MDSC from peripheral lymphoid organs suppressed antigen-specific CD8(+) T cells but failed to inhibit nonspecific T cell function. In sharp contrast, tumor MDSC suppressed both antigen-specific and nonspecific T cell activity. The tumor microenvironment caused rapid and dramatic up-regulation of arginase I and inducible nitric oxide synthase in MDSC, which was accompanied by down-regulation of nicotinamide adenine dinucleotide phosphate-oxidase and reactive oxygen species in these cells. In contrast to MDSC from the spleen, MDSC from the tumor site rapidly differentiated into macrophages. Exposure of spleen MDSC to hypoxia resulted in the conversion of these cells to nonspecific suppressors and their preferential differentiation to macrophages. Hypoxia-inducible factor (HIF) 1α was found to be primarily responsible for the observed effects of the tumor microenvironment on MDSC differentiation and function. Thus, hypoxia via HIF-1α dramatically alters the function of MDSC in the tumor microenvironment and redirects their differentiation toward tumor-associated macrophages, hence providing a mechanistic link between different myeloid suppressive cells in the tumor microenvironment.
Figures

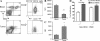

Similar articles
-
PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation.J Exp Med. 2014 May 5;211(5):781-90. doi: 10.1084/jem.20131916. Epub 2014 Apr 28. J Exp Med. 2014. PMID: 24778419 Free PMC article.
-
Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14⁻/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer.J Cancer Res Clin Oncol. 2010 Jan;136(1):35-45. doi: 10.1007/s00432-009-0634-0. J Cancer Res Clin Oncol. 2010. PMID: 19572148
-
Tumor-Promoting Effects of Myeloid-Derived Suppressor Cells Are Potentiated by Hypoxia-Induced Expression of miR-210.Cancer Res. 2015 Sep 15;75(18):3771-87. doi: 10.1158/0008-5472.CAN-15-0405. Epub 2015 Jul 23. Cancer Res. 2015. PMID: 26206559
-
Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy.Oncoimmunology. 2015 Feb 3;4(1):e954829. doi: 10.4161/21624011.2014.954829. eCollection 2015 Jan. Oncoimmunology. 2015. PMID: 25949858 Free PMC article. Review.
-
Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment.Immunology. 2014 Dec;143(4):512-9. doi: 10.1111/imm.12380. Immunology. 2014. PMID: 25196648 Free PMC article. Review.
Cited by
-
Hypoxia Supports Differentiation of Terminally Exhausted CD8 T Cells.Front Immunol. 2021 May 7;12:660944. doi: 10.3389/fimmu.2021.660944. eCollection 2021. Front Immunol. 2021. PMID: 34025660 Free PMC article.
-
Lack of the Transcription Factor Hypoxia-Inducible Factor 1α (HIF-1α) in Macrophages Accelerates the Necrosis of Mycobacterium avium-Induced Granulomas.Infect Immun. 2015 Sep;83(9):3534-44. doi: 10.1128/IAI.00144-15. Epub 2015 Jun 22. Infect Immun. 2015. PMID: 26099585 Free PMC article.
-
Myeloid-derived suppressor cells in multiple myeloma: pre-clinical research and translational opportunities.Front Oncol. 2014 Dec 8;4:348. doi: 10.3389/fonc.2014.00348. eCollection 2014. Front Oncol. 2014. PMID: 25538892 Free PMC article. Review.
-
Underlying mechanisms and drug intervention strategies for the tumour microenvironment.J Exp Clin Cancer Res. 2021 Mar 15;40(1):97. doi: 10.1186/s13046-021-01893-y. J Exp Clin Cancer Res. 2021. PMID: 33722297 Free PMC article. Review.
-
The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells.Immunology. 2013 Feb;138(2):105-15. doi: 10.1111/imm.12036. Immunology. 2013. PMID: 23216602 Free PMC article. Review.
References
-
- Almand B., Clark J.I., Nikitina E., van Beynen J., English N.R., Knight S.C., Carbone D.P., Gabrilovich D.I. 2001. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166:678–689 - PubMed
-
- Cheng P., Corzo C.A., Luetteke N., Yu B., Nagaraj S., Bui M.M., Ortiz M., Nacken W., Sorg C., Vogl T., et al. 2008. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205:2235–2249 10.1084/jem.20080132 - DOI - PMC - PubMed
-
- Corzo C.A., Cotter M.J., Cheng P., Cheng F., Kusmartsev S., Sotomayor E., Padhya T., McCaffrey T.V., McCaffrey J.C., Gabrilovich D.I. 2009. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182:5693–5701 10.4049/jimmunol.0900092 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

