Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis
- PMID: 20876282
- PMCID: PMC2953436
- DOI: 10.1083/jcb.200911143
Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis
Abstract
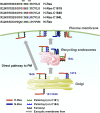
Ras proteins regulate cell growth, death, and differentiation, and it is well established that this functional versatility is accomplished through their different subcellular localizations. Palmitoylated H- and N-Ras are believed to localize at the perinuclear Golgi and plasma membrane (PM). Notably, however, recycling endosomes (REs) also localize to a perinuclear region, which is often indistinguishable from the Golgi. In this study, we show that active palmitoylated Ras proteins mainly localize intracellularly at REs and that REs act as a way station along the post-Golgi exocytic pathway to the PM. H-Ras requires two palmitoyl groups for RE targeting. The lack of either or both palmitoyl groups leads to the mislocalization of the mutant proteins to the endoplasmic reticulum, Golgi apparatus, or the PM. Therefore, we demonstrate that palmitoylation directs Ras proteins to the correct intracellular organelles for trafficking and activity.
Figures
Similar articles
-
Palmitoylation pilots ras to recycling endosomes.Small GTPases. 2011 Mar;2(2):82-84. doi: 10.4161/sgtp.2.2.15245. Small GTPases. 2011. PMID: 21776406 Free PMC article.
-
H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway.Mol Cell Biol. 2000 Apr;20(7):2475-87. doi: 10.1128/MCB.20.7.2475-2487.2000. Mol Cell Biol. 2000. PMID: 10713171 Free PMC article.
-
H-Ras dynamically interacts with recycling endosomes in CHO-K1 cells: involvement of Rab5 and Rab11 in the trafficking of H-Ras to this pericentriolar endocytic compartment.J Biol Chem. 2005 Oct 14;280(41):34997-5010. doi: 10.1074/jbc.M506256200. Epub 2005 Aug 3. J Biol Chem. 2005. PMID: 16079139
-
Compartmentalized signalling: Ras proteins and signalling nanoclusters.FEBS J. 2009 Apr;276(7):1817-25. doi: 10.1111/j.1742-4658.2009.06928.x. Epub 2009 Feb 23. FEBS J. 2009. PMID: 19243428 Review.
-
Life and Death of Fungal Transporters under the Challenge of Polarity.Int J Mol Sci. 2020 Jul 29;21(15):5376. doi: 10.3390/ijms21155376. Int J Mol Sci. 2020. PMID: 32751072 Free PMC article. Review.
Cited by
-
Acylpeptide hydrolase is a novel regulator of KRAS plasma membrane localization and function.J Cell Sci. 2019 Jul 31;132(15):jcs232132. doi: 10.1242/jcs.232132. J Cell Sci. 2019. PMID: 31266814 Free PMC article.
-
Recycling endosomes associate with Golgi stacks in sea urchin embryos.Commun Integr Biol. 2020 Apr 30;13(1):59-62. doi: 10.1080/19420889.2020.1761069. eCollection 2020. Commun Integr Biol. 2020. PMID: 32395196 Free PMC article.
-
The palmitoylation of the N-terminal extracellular Cys37 mediates the nuclear translocation of VPAC1 contributing to its anti-apoptotic activity.Oncotarget. 2017 Jun 27;8(26):42728-42741. doi: 10.18632/oncotarget.17449. Oncotarget. 2017. PMID: 28473666 Free PMC article.
-
Escorting Ras.Small GTPases. 2012 Oct-Dec;3(4):236-9. doi: 10.4161/sgtp.20460. Epub 2012 Jun 27. Small GTPases. 2012. PMID: 22735486 Free PMC article.
-
Regulating the regulator: post-translational modification of RAS.Nat Rev Mol Cell Biol. 2011 Dec 22;13(1):39-51. doi: 10.1038/nrm3255. Nat Rev Mol Cell Biol. 2011. PMID: 22189424 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

