Dachshund homologues play a conserved role in islet cell development
- PMID: 20869363
- PMCID: PMC2997432
- DOI: 10.1016/j.ydbio.2010.09.007
Dachshund homologues play a conserved role in islet cell development
Abstract
All metazoans use insulin to control energy metabolism, but they secrete it from different cells: neurons in the central nervous system in invertebrates and endocrine cells in the gut or pancreas in vertebrates. Despite their origins in different germ layers, all of these insulin-producing cells share common functional features and gene expression patterns. In this study, we tested the role in insulin-producing cells of the vertebrate homologues of Dachshund, a transcriptional regulator that marks the earliest committed progenitors of the neural insulin-producing cells in Drosophila. Both zebrafish and mice expressed a single dominant Dachshund homologue in the pancreatic endocrine lineage, and in both species loss of this homologue reduced the numbers of all islet cell types including the insulin-producing β-cells. In mice, Dach1 gene deletion left the pancreatic progenitor cells unaltered, but blocked the perinatal burst of proliferation of differentiated β-cells that normally generates most of the β-cell mass. In β-cells, Dach1 bound to the promoter of the cell cycle inhibitor p27Kip1, which constrains β-cell proliferation. Taken together, these data demonstrate a conserved role for Dachshund homologues in the production of insulin-producing cells.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

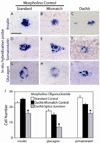
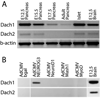
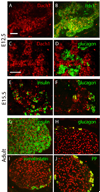
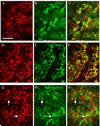
Similar articles
-
Exdpf is a key regulator of exocrine pancreas development controlled by retinoic acid and ptf1a in zebrafish.PLoS Biol. 2008 Nov 25;6(11):e293. doi: 10.1371/journal.pbio.0060293. PLoS Biol. 2008. PMID: 19067490 Free PMC article.
-
Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development.Development. 2010 Jan;137(2):203-12. doi: 10.1242/dev.041673. Development. 2010. PMID: 20040487 Free PMC article.
-
Zebrafish mnx1 controls cell fate choice in the developing endocrine pancreas.Development. 2011 Nov;138(21):4597-608. doi: 10.1242/dev.067736. Development. 2011. PMID: 21989909 Free PMC article.
-
Development and Characteristics of Pancreatic Epsilon Cells.Int J Mol Sci. 2019 Apr 16;20(8):1867. doi: 10.3390/ijms20081867. Int J Mol Sci. 2019. PMID: 31014006 Free PMC article. Review.
-
The beta cell transcription factors and development of the pancreas.J Mol Med (Berl). 1997 May;75(5):327-40. doi: 10.1007/s001090050118. J Mol Med (Berl). 1997. PMID: 9181474 Review.
Cited by
-
The transcription factor Dach1 is essential for podocyte function.J Cell Mol Med. 2018 May;22(5):2656-2669. doi: 10.1111/jcmm.13544. Epub 2018 Mar 2. J Cell Mol Med. 2018. PMID: 29498212 Free PMC article.
-
Familial young-onset diabetes, pre-diabetes and cardiovascular disease are associated with genetic variants of DACH1 in Chinese.PLoS One. 2014 Jan 20;9(1):e84770. doi: 10.1371/journal.pone.0084770. eCollection 2014. PLoS One. 2014. PMID: 24465431 Free PMC article.
-
Concise review: in search of unlimited sources of functional human pancreatic beta cells.Stem Cells Transl Med. 2013 Jan;2(1):61-7. doi: 10.5966/sctm.2012-0120. Epub 2012 Dec 19. Stem Cells Transl Med. 2013. PMID: 23283495 Free PMC article. Review.
-
Integrative single-cell characterization of a frugivorous and an insectivorous bat kidney and pancreas.Nat Commun. 2024 Jan 9;15(1):12. doi: 10.1038/s41467-023-44186-y. Nat Commun. 2024. PMID: 38195585 Free PMC article.
-
Insects as a New Complex Model in Hormonal Basis of Obesity.Int J Mol Sci. 2021 Oct 14;22(20):11066. doi: 10.3390/ijms222011066. Int J Mol Sci. 2021. PMID: 34681728 Free PMC article. Review.
References
-
- Argenton F, Zecchin E, Bortolussi M. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech Dev. 1999;87:217–221. - PubMed
-
- Backman M, Machon O, Van Den Bout CJ, Krauss S. Targeted disruption of mouse Dach1 results in postnatal lethality. Dev Dyn. 2003;226:139–144. - PubMed
-
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Developmental Biology. 2001;230:189–203. - PubMed
-
- Caubit X, Thangarajah R, Theil T, Wirth J, Nothwang HG, Ruther U, Krauss S. Mouse Dac, a novel nuclear factor with homology to Drosophila dachshund shows a dynamic expression in the neural crest, the eye, the neocortex, and the limb bud. Dev Dyn. 1999;214:66–80. - PubMed
-
- Cordes SP. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin Genet. 2005;68:487–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK063720-02/DK/NIDDK NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- R01 DK021344/DK/NIDDK NIH HHS/United States
- R01 DK021344-28/DK/NIDDK NIH HHS/United States
- P30 DK063720-05/DK/NIDDK NIH HHS/United States
- P30 DK063720-04/DK/NIDDK NIH HHS/United States
- R01 DK021344-25/DK/NIDDK NIH HHS/United States
- U19 DK061245/DK/NIDDK NIH HHS/United States
- P30 DK063720-03/DK/NIDDK NIH HHS/United States
- P30 DK063720-07/DK/NIDDK NIH HHS/United States
- R01 DK021344-27A2/DK/NIDDK NIH HHS/United States
- R01DK075032/DK/NIDDK NIH HHS/United States
- P30 DK063720-06A1/DK/NIDDK NIH HHS/United States
- U19 DK061245-04/DK/NIDDK NIH HHS/United States
- R01 DK075032/DK/NIDDK NIH HHS/United States
- U19 DK61245/DK/NIDDK NIH HHS/United States
- R01 DK021344-26/DK/NIDDK NIH HHS/United States
- R01DK21344/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

