TRPV1 in brain is involved in acetaminophen-induced antinociception
- PMID: 20862299
- PMCID: PMC2941447
- DOI: 10.1371/journal.pone.0012748
TRPV1 in brain is involved in acetaminophen-induced antinociception
Abstract
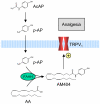
Background: Acetaminophen, the major active metabolite of acetanilide in man, has become one of the most popular over-the-counter analgesic and antipyretic agents, consumed by millions of people daily. However, its mechanism of action is still a matter of debate. We have previously shown that acetaminophen is further metabolized to N-(4-hydroxyphenyl)-5Z,8Z,11Z,14Z -eicosatetraenamide (AM404) by fatty acid amide hydrolase (FAAH) in the rat and mouse brain and that this metabolite is a potent activator of transient receptor potential vanilloid 1 (TRPV(1)) in vitro. Pharmacological activation of TRPV(1) in the midbrain periaqueductal gray elicits antinociception in rats. It is therefore possible that activation of TRPV(1) in the brain contributes to the analgesic effect of acetaminophen.
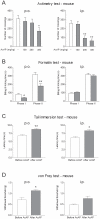
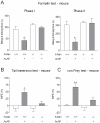
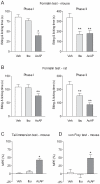
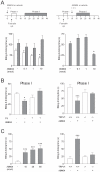
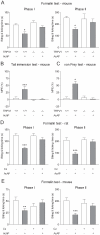
Methodology/principal findings: Here we show that the antinociceptive effect of acetaminophen at an oral dose lacking hypolocomotor activity is absent in FAAH and TRPV(1) knockout mice in the formalin, tail immersion and von Frey tests. This dose of acetaminophen did not affect the global brain contents of prostaglandin E(2) (PGE(2)) and endocannabinoids. Intracerebroventricular injection of AM404 produced a TRPV(1)-mediated antinociceptive effect in the mouse formalin test. Pharmacological inhibition of TRPV(1) in the brain by intracerebroventricular capsazepine injection abolished the antinociceptive effect of oral acetaminophen in the same test.
Conclusions: This study shows that TRPV(1) in brain is involved in the antinociceptive action of acetaminophen and provides a strategy for developing central nervous system active oral analgesics based on the coexpression of FAAH and TRPV(1) in the brain.
Conflict of interest statement
Figures
Similar articles
-
Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain.PLoS One. 2013 Aug 5;8(8):e70690. doi: 10.1371/journal.pone.0070690. Print 2013. PLoS One. 2013. PMID: 23940628 Free PMC article.
-
Paracetamol analogues conjugated by FAAH induce TRPV1-mediated antinociception without causing acute liver toxicity.Eur J Med Chem. 2021 Mar 5;213:113042. doi: 10.1016/j.ejmech.2020.113042. Epub 2020 Nov 21. Eur J Med Chem. 2021. PMID: 33257173
-
Activation of the capsaicin-receptor TRPV1 by the acetaminophen metabolite N-arachidonoylaminophenol results in cytotoxicity.Life Sci. 2018 Feb 1;194:67-74. doi: 10.1016/j.lfs.2017.12.024. Epub 2017 Dec 19. Life Sci. 2018. PMID: 29273526
-
Receptor and Molecular Targets for the Development of Novel Opioid and Non-Opioid Analgesic Therapies.Pain Physician. 2021 Mar;24(2):153-163. Pain Physician. 2021. PMID: 33740349 Review.
-
Central antinociceptive effects of non-steroidal anti-inflammatory drugs and paracetamol. Experimental studies in the rat.Acta Anaesthesiol Scand Suppl. 1995;103:1-44. Acta Anaesthesiol Scand Suppl. 1995. PMID: 7725891 Review.
Cited by
-
Electroacupuncture Reduced Fibromyalgia-Pain-like Behavior through Inactivating Transient Receptor Potential V1 and Interleukin-17 in Intermittent Cold Stress Mice Model.Brain Sci. 2024 Aug 28;14(9):869. doi: 10.3390/brainsci14090869. Brain Sci. 2024. PMID: 39335365 Free PMC article.
-
Premedication with oral paracetamol for reduction of propofol injection pain: a randomized placebo-controlled trial.BMC Anesthesiol. 2019 Jun 11;19(1):100. doi: 10.1186/s12871-019-0758-y. BMC Anesthesiol. 2019. PMID: 31185906 Free PMC article. Clinical Trial.
-
Long-term adverse effects of paracetamol - a review.Br J Clin Pharmacol. 2018 Oct;84(10):2218-2230. doi: 10.1111/bcp.13656. Epub 2018 Jul 20. Br J Clin Pharmacol. 2018. PMID: 29863746 Free PMC article. Review.
-
Acetaminophen Relieves Inflammatory Pain through CB1 Cannabinoid Receptors in the Rostral Ventromedial Medulla.J Neurosci. 2018 Jan 10;38(2):322-334. doi: 10.1523/JNEUROSCI.1945-17.2017. Epub 2017 Nov 22. J Neurosci. 2018. PMID: 29167401 Free PMC article.
-
The analgesic efficacy of intra-articular acetaminophen in an experimental model of carrageenan-induced arthritis.Pain Res Manag. 2013 Sep-Oct;18(5):e63-7. doi: 10.1155/2013/148392. Pain Res Manag. 2013. PMID: 24093120 Free PMC article.
References
-
- Brodie BB, Axelrod J. The fate of acetanilide in man. J Pharmacol Exp Ther. 1948;94:29–38. - PubMed
-
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110:1170–1179. - PubMed
-
- Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. - PubMed
-
- Bujalska M, Gumulka WS. Effect of cyclooxygenase and NO synthase inhibitors on antinociceptive action of acetaminophen. Pol J Pharmacol. 2001;53:341–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

