Maternal embryonic leucine zipper kinase is upregulated and required in mammary tumor-initiating cells in vivo
- PMID: 20861186
- PMCID: PMC3990264
- DOI: 10.1158/0008-5472.CAN-10-1295
Maternal embryonic leucine zipper kinase is upregulated and required in mammary tumor-initiating cells in vivo
Abstract
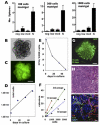
Maternal embryonic leucine zipper kinase (MELK) is expressed in several developing tissues, in the adult germ line, and in adult neural progenitors. MELK expression is elevated in aggressive undifferentiated tumors, correlating with poor patient outcome in human breast cancer. To investigate the role of MELK in mammary tumorigenesis in vivo, we used a MELK-green fluorescent protein (GFP) reporter mouse, which allows prospective isolation of MELK-expressing cells based on GFP fluorescence. We found that in the normal mammary gland, cells expressing high levels of MELK were enriched in proliferating cells that express markers of mammary progenitors. The isolation of cells with high levels of MELK in mammary tumors from MMTV-Wnt1/MELK-GFP bitransgenic mice resulted in a significant enrichment of tumorsphere formation in culture and tumor initiation after transplantation into mammary fat pads of syngeneic mice. Furthermore, using lentiviral delivery of MELK-specific shRNA and limiting dilution cell transplantations, we showed that MELK function is required for mammary tumorigenesis in vivo. Our findings identify MELK as a potential target in breast tumor-initiating cells.
©2010 AACR.
Figures
Similar articles
-
Tumor-initiating function of nucleostemin-enriched mammary tumor cells.Cancer Res. 2010 Nov 15;70(22):9444-52. doi: 10.1158/0008-5472.CAN-10-2159. Epub 2010 Nov 2. Cancer Res. 2010. PMID: 21045149 Free PMC article.
-
Cooperative signaling between Wnt1 and integrin-linked kinase induces accelerated breast tumor development.Breast Cancer Res. 2010;12(3):R38. doi: 10.1186/bcr2592. Epub 2010 Jun 21. Breast Cancer Res. 2010. PMID: 20565980 Free PMC article.
-
Wnt5a suppresses tumor formation and redirects tumor phenotype in MMTV-Wnt1 tumors.PLoS One. 2014 Nov 17;9(11):e113247. doi: 10.1371/journal.pone.0113247. eCollection 2014. PLoS One. 2014. PMID: 25401739 Free PMC article.
-
Maternal embryonic leucine zipper kinase: key kinase for stem cell phenotype in glioma and other cancers.Mol Cancer Ther. 2014 Jun;13(6):1393-8. doi: 10.1158/1535-7163.MCT-13-0764. Epub 2014 May 2. Mol Cancer Ther. 2014. PMID: 24795222 Free PMC article. Review.
-
Maternal embryonic leucine zipper kinase in tumor cells and tumor microenvironment: An emerging player and promising therapeutic opportunity.Cancer Lett. 2023 Apr 28;560:216126. doi: 10.1016/j.canlet.2023.216126. Epub 2023 Mar 16. Cancer Lett. 2023. PMID: 36933780 Review.
Cited by
-
Differential network analysis applied to preoperative breast cancer chemotherapy response.PLoS One. 2013 Dec 9;8(12):e81784. doi: 10.1371/journal.pone.0081784. eCollection 2013. PLoS One. 2013. PMID: 24349128 Free PMC article.
-
Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery.Cell. 2020 Apr 2;181(1):102-114. doi: 10.1016/j.cell.2019.11.031. Epub 2020 Jan 16. Cell. 2020. PMID: 31955850 Free PMC article. Review.
-
Maternal embryonic leucine zipper kinase enhances gastric cancer progression via the FAK/Paxillin pathway.Mol Cancer. 2014 May 4;13:100. doi: 10.1186/1476-4598-13-100. Mol Cancer. 2014. PMID: 24885567 Free PMC article.
-
Cortical localization of maternal embryonic leucine zipper kinase (MELK) implicated in cytokinesis in early xenopus embryos.Commun Integr Biol. 2011 Jul;4(4):483-5. doi: 10.4161/cib.4.4.15669. Epub 2011 Jul 1. Commun Integr Biol. 2011. PMID: 21966578 Free PMC article.
-
MELK is an oncogenic kinase essential for mitotic progression in basal-like breast cancer cells.Elife. 2014 May 20;3:e01763. doi: 10.7554/eLife.01763. Elife. 2014. PMID: 24844244 Free PMC article.
References
-
- Stevens LC. Studies on transplantable testicular teratomas of strain 129 mice. Journal of the National Cancer Institute. 1958;20:1257–75. - PubMed
-
- Kleinsmith LJ, Pierce GB., Jr Multipotentiality of Single Embryonal Carcinoma Cells. Cancer research. 1964;24:1544–51. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. - PubMed
-
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

