Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors
- PMID: 20855968
- PMCID: PMC3047092
- DOI: 10.4161/cbt.10.10.13251
Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors
Abstract
Background: Single-dose infusion of the agonistic anti-CD40 monoclonal antibody (mAb) CP-870,893 accomplishes immune activation and clinical responses in patients with advanced cancers, but repeat dosing of this agent has not been reported.
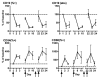
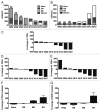
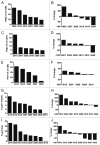
Results: Twenty-seven patients were enrolled. The most common adverse event was transient, infusion-related cytokine release syndrome (CRS). Dose-limiting toxicities included grade 3 CRS and grade 3 urticaria; the maximum tolerated dose (MTD) was estimated to be 0.2 mg/kg. Seven patients (26%) had stable disease as the best clinical response; no partial or complete responses were observed. At the MTD, patient B lymphocytes exhibited persistently increased expression of costimulatory and adhesion molecules without resetting to baseline between doses. In 4 of 8 patients (50%) evaluated at the MTD, there were marked declines in total CD3(+) T lymphocytes, as well as CD4(+) and CD8(+) subsets.
Patients and methods: Patients with advanced solid tumor malignancies received weekly intravenous infusions of CP-870,893 in four dose level cohorts. Safety and immune pharmacodynamics were assessed.
Conclusions: Weekly infusions of the agonist CD40 antibody CP-870,893 were well-tolerated, but there was little clinical activity in advanced cancer patients. Correlative studies demonstrate chronic B cell activation and in some patients, T cell depletion. Longer dosing intervals may be desirable for optimal immune pharmacodynamics.
Figures

Comment in
-
Poking CD40 for cancer therapy, another example of the Goldilocks effect.Cancer Biol Ther. 2010 Nov 15;10(10):994-6. doi: 10.4161/cbt.10.10.13976. Epub 2010 Nov 15. Cancer Biol Ther. 2010. PMID: 21057206 No abstract available.
Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
A phase I dose-escalation study of LRP5/6 antagonist BI 905677 in patients with advanced solid tumors.ESMO Open. 2024 Nov;9(11):103729. doi: 10.1016/j.esmoop.2024.103729. ESMO Open. 2024. PMID: 39617530 Free PMC article. Clinical Trial.
-
Interventions for preventing weight gain after smoking cessation.Cochrane Database Syst Rev. 2021 Oct 6;10(10):CD006219. doi: 10.1002/14651858.CD006219.pub4. Cochrane Database Syst Rev. 2021. PMID: 34611902 Free PMC article. Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
Pre-clinical evaluation of immunoPET imaging using agonist CD40 monoclonal antibody in pancreatic tumor-bearing mice.Nucl Med Biol. 2021 Jul-Aug;98-99:8-17. doi: 10.1016/j.nucmedbio.2021.04.001. Epub 2021 Apr 21. Nucl Med Biol. 2021. PMID: 33962357 Free PMC article.
-
A bedside to bench study of anti-PD-1, anti-CD40, and anti-CSF1R indicates that more is not necessarily better.Mol Cancer. 2023 Nov 14;22(1):182. doi: 10.1186/s12943-023-01884-x. Mol Cancer. 2023. PMID: 37964379 Free PMC article.
-
The human anti-CD40 agonist antibody mitazalimab (ADC-1013; JNJ-64457107) activates antigen-presenting cells, improves expansion of antigen-specific T cells, and enhances anti-tumor efficacy of a model cancer vaccine in vivo.Cancer Immunol Immunother. 2021 Dec;70(12):3629-3642. doi: 10.1007/s00262-021-02932-5. Epub 2021 May 5. Cancer Immunol Immunother. 2021. PMID: 33948686 Free PMC article.
-
Latest Advances in Targeting the Tumor Microenvironment for Tumor Suppression.Int J Mol Sci. 2019 Sep 23;20(19):4719. doi: 10.3390/ijms20194719. Int J Mol Sci. 2019. PMID: 31547627 Free PMC article. Review.
-
A CD40 Agonist and PD-1 Antagonist Antibody Reprogram the Microenvironment of Nonimmunogenic Tumors to Allow T-cell-Mediated Anticancer Activity.Cancer Immunol Res. 2019 Mar;7(3):428-442. doi: 10.1158/2326-6066.CIR-18-0061. Epub 2019 Jan 14. Cancer Immunol Res. 2019. PMID: 30642833 Free PMC article.
References
-
- Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–1088. - PubMed
-
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. - PubMed
-
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T- helper and a T-killer cell. Nature. 1998;393:474–478. - PubMed
-
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. - PubMed
-
- French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
