Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer
- PMID: 20855838
- PMCID: PMC2974340
- DOI: 10.1200/JCO.2010.30.7025
Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer
Abstract
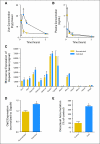
Purpose: To assess whether the low incidence of severe neutropenia in castrated men with prostate cancer treated with docetaxel is the result of changes in systemic clearance.
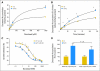
Patients and methods: A total of 10 noncastrated and 20 castrated men with prostate cancer were studied to achieve 80% power (α = .05) to detect at least a 25% change in the clearance of docetaxel. The erythromycin breath test was evaluated to determine hepatic activity of cytochrome P450 3A4 (CYP3A4), the main docetaxel-metabolizing enzyme. Additional studies were performed in rats and transfected cells overexpressing human or rodent transporters.
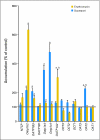
Results: Docetaxel clearance was increased by approximately 100% in castrated men and was associated with a two-fold reduction in area under the curve (P = .0001), although hepatic activity of CYP3A4 was unchanged (P = .26). In rats, castration was associated with higher uptake of docetaxel in the liver and a concurrent increase in the expression of rOat2 (Slc22a7), an organic anion transporter that regulates, in part, the transfer of docetaxel from the circulation into hepatocytes.
Conclusion: It is recommended that castration- and/or hormone-related changes in the clearance of oncology drugs should be considered as a possible risk factor for treatment failure.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Castration-dependent pharmacokinetics of docetaxel: do sex and/or ABCB1 polymorphism also matter?J Clin Oncol. 2011 May 20;29(15):e454-5; author reply e456-7. doi: 10.1200/JCO.2010.34.5439. Epub 2011 Apr 11. J Clin Oncol. 2011. PMID: 21482999 No abstract available.
Similar articles
-
Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance.Mol Cancer Ther. 2015 Apr;14(4):994-1003. doi: 10.1158/1535-7163.MCT-14-0547. Epub 2015 Feb 18. Mol Cancer Ther. 2015. PMID: 25695959 Free PMC article.
-
Docetaxel pharmacokinetics and its correlation with two in vivo probes for cytochrome P450 enzymes: the C(14)-erythromycin breath test and the antipyrine clearance test.Cancer Chemother Pharmacol. 2012 Jan;69(1):125-35. doi: 10.1007/s00280-011-1676-y. Epub 2011 May 28. Cancer Chemother Pharmacol. 2012. PMID: 21626050 Clinical Trial.
-
The relationship of polymorphisms in ABCC2 and SLCO1B3 with docetaxel pharmacokinetics and neutropenia: CALGB 60805 (Alliance).Pharmacogenet Genomics. 2013 Jan;23(1):29-33. doi: 10.1097/FPC.0b013e32835b16d8. Pharmacogenet Genomics. 2013. PMID: 23188068 Free PMC article.
-
Optimizing the use of docetaxel in men with castration-resistant metastatic prostate cancer.Prostate Cancer Prostatic Dis. 2010 Jun;13(2):108-16. doi: 10.1038/pcan.2009.62. Epub 2010 Jan 12. Prostate Cancer Prostatic Dis. 2010. PMID: 20066005 Review.
-
New agents for the management of castration-resistant prostate cancer.Ann Pharmacother. 2012 Nov;46(11):1518-28. doi: 10.1345/aph.1R169. Epub 2012 Nov 7. Ann Pharmacother. 2012. PMID: 23136351 Review.
Cited by
-
Solute Carrier Transportome in Chemotherapy-Induced Adverse Drug Reactions.Rev Physiol Biochem Pharmacol. 2022;183:177-215. doi: 10.1007/112_2020_30. Rev Physiol Biochem Pharmacol. 2022. PMID: 32761456 Free PMC article.
-
Uptake carriers and oncology drug safety.Drug Metab Dispos. 2014 Apr;42(4):611-22. doi: 10.1124/dmd.113.055806. Epub 2013 Dec 30. Drug Metab Dispos. 2014. PMID: 24378324 Free PMC article. Review.
-
Prognostic role of docetaxel-induced suppression of free testosterone serum levels in metastatic prostate cancer patients.Sci Rep. 2021 Aug 12;11(1):16457. doi: 10.1038/s41598-021-95874-y. Sci Rep. 2021. PMID: 34385568 Free PMC article.
-
Association of prostate cancer SLCO gene expression with Gleason grade and alterations following androgen deprivation therapy.Prostate Cancer Prostatic Dis. 2019 Dec;22(4):560-568. doi: 10.1038/s41391-019-0141-6. Epub 2019 Mar 19. Prostate Cancer Prostatic Dis. 2019. PMID: 30890759 Free PMC article.
-
Multidisciplinary total eradication therapy (TET) in men with newly diagnosed oligometastatic prostate cancer.Med Oncol. 2020 Jun 10;37(7):60. doi: 10.1007/s12032-020-01385-7. Med Oncol. 2020. PMID: 32524295 Free PMC article.
References
-
- Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel: Recent developments. Clin Pharmacokinet. 2006;45:235–252. - PubMed
-
- Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. - PubMed
-
- Bruno R, Olivares R, Berille J, et al. Alpha-1-acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9:1077–1082. - PubMed
-
- sanofi-aventis: Product insert for Taxotere (docetaxel) http://products.sanofi-aventis.us/Taxotere/taxotere.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

