Obesity and leptin resistance: distinguishing cause from effect
- PMID: 20846876
- PMCID: PMC2967652
- DOI: 10.1016/j.tem.2010.08.002
Obesity and leptin resistance: distinguishing cause from effect
Abstract
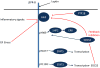
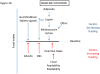
Because leptin reduces food intake and body weight, the coexistence of elevated leptin levels with obesity is widely interpreted as evidence of 'leptin resistance.' Indeed, obesity promotes a number of cellular processes that attenuate leptin signaling (referred to here as 'cellular leptin resistance') and amplify the extent of weight gain induced by genetic and environmental factors. As commonly used, however, the term 'leptin resistance' embraces a range of phenomena that are distinct in underlying mechanisms and pathophysiological implications. Moreover, the induction of cellular leptin resistance by obesity complicates efforts to distinguish the mechanisms that predispose to weight gain from those that result from it. We suggest a framework for approaching these issues and important avenues for future investigation.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts: RJS receives research support, consults, and is on the speakers’ bureau for Amylin Pharmaceuticals, and is on the speakers’ bureau and scientific advisory board for Novo Nordisk.
Figures


Similar articles
-
Central leptin receptor action and resistance in obesity.J Investig Med. 2009 Oct;57(7):789-94. doi: 10.2310/JIM.0b013e3181bb0d49. J Investig Med. 2009. PMID: 20029269 Free PMC article. Review.
-
Leptin resistance and obesity.Endocr J. 2007 Feb;54(1):17-26. doi: 10.1507/endocrj.kr-85. Epub 2006 Oct 20. Endocr J. 2007. PMID: 17053294 Review. No abstract available.
-
Leptin-signaling pathways and leptin resistance.Forum Nutr. 2010;63:123-132. doi: 10.1159/000264400. Epub 2009 Nov 27. Forum Nutr. 2010. PMID: 19955780 Free PMC article. Review.
-
A mathematical model of leptin resistance.Math Biosci. 2015 Sep;267:10-23. doi: 10.1016/j.mbs.2015.06.008. Epub 2015 Jun 23. Math Biosci. 2015. PMID: 26116428
-
Phenomenon of leptin resistance in seasonal animals: the failure of leptin action in the brain.Domest Anim Endocrinol. 2015 Jul;52:60-70. doi: 10.1016/j.domaniend.2015.03.002. Epub 2015 Mar 14. Domest Anim Endocrinol. 2015. PMID: 25863197 Review.
Cited by
-
Effects of leptin replacement alone and with exendin-4 on food intake and weight regain in weight-reduced diet-induced obese rats.Am J Physiol Endocrinol Metab. 2012 Jun 15;302(12):E1576-85. doi: 10.1152/ajpendo.00058.2012. Epub 2012 Apr 17. Am J Physiol Endocrinol Metab. 2012. PMID: 22510712 Free PMC article.
-
Synaptic plasticity in neuronal circuits regulating energy balance.Nat Neurosci. 2012 Oct;15(10):1336-42. doi: 10.1038/nn.3219. Epub 2012 Sep 25. Nat Neurosci. 2012. PMID: 23007188 Review.
-
Dietary components in the development of leptin resistance.Adv Nutr. 2013 Mar 1;4(2):164-75. doi: 10.3945/an.112.003152. Adv Nutr. 2013. PMID: 23493533 Free PMC article. Review.
-
Is There A Causal Relationship between Childhood Obesity and Acute Lymphoblastic Leukemia? A Review.Cancers (Basel). 2020 Oct 22;12(11):3082. doi: 10.3390/cancers12113082. Cancers (Basel). 2020. PMID: 33105727 Free PMC article. Review.
-
The role of leptin and low testosterone in obesity.Int J Impot Res. 2022 Nov;34(7):704-713. doi: 10.1038/s41443-022-00534-y. Epub 2022 Jan 31. Int J Impot Res. 2022. PMID: 35102263 Review.
References
-
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. - PubMed
-
- Rosenbaum M, Leibel RL. The role of leptin in human physiology. N Engl J Med. 1999;341:913–915. - PubMed
-
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. - PubMed
-
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK057768/DK/NIDDK NIH HHS/United States
- R01 DK090320/DK/NIDDK NIH HHS/United States
- R01 DK057768/DK/NIDDK NIH HHS/United States
- R01 DK083042/DK/NIDDK NIH HHS/United States
- R37 DK056731/DK/NIDDK NIH HHS/United States
- DK52431/DK/NIDDK NIH HHS/United States
- DK052989/DK/NIDDK NIH HHS/United States
- R01 DK078056/DK/NIDDK NIH HHS/United States
- DK083042/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R01 DK052431/DK/NIDDK NIH HHS/United States
- DK056731/DK/NIDDK NIH HHS/United States
- R01 DK056731/DK/NIDDK NIH HHS/United States
- R01 DK052989/DK/NIDDK NIH HHS/United States
- DK078056/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

