Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia
- PMID: 20840857
- PMCID: PMC2956789
- DOI: 10.1016/j.bbamcr.2010.09.002
Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia
Abstract
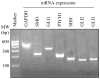
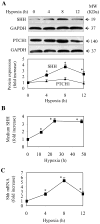
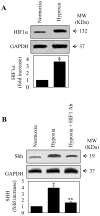
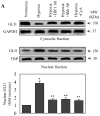
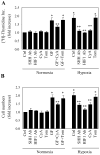
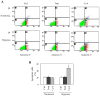
The hedgehog signal pathway plays a crucial role in the angiogenesis and vascular remodeling. However, the function of this pathway in the pulmonary vascular smooth cell proliferation in response to hypoxia remains unknown. In this study, we have demonstrated that the main components of the hedgehog pathway, including sonic hedgehog (SHH), patched1 (PTCH1), smoothened (SMO), GLI and hypoxia-inducible factor 1 (HIF1) are expressed in the human pulmonary arterial smooth muscle cells (HPASMCs). Interestingly, hypoxia significantly enhanced the expression of SHH and HIF1, facilitated the translocation of GLI1 into the nuclei, and promoted the proliferation of HPASMCs. Furthermore, direct activation of the SHH pathway through incubation with the purified recombinant human SHH or with purmorphamine and SAG, two Smo agonists, also enhanced the proliferation of HPASMCs. Importantly, the treatment with anti-SHH and anti-HIF1 antibodies or cyclopamine, a specific SMO inhibitor, markedly inhibited the nuclear translocation of GLI1 and cell proliferation in the HPASMCs induced by hypoxia and activation of the SHH pathway. Moreover, the treatment with cyclopamine increased apoptosis in the hypoxic HPASMCs. These data strongly demonstrate for the first time that the SHH signaling plays a crucial role in the regulation of HPASMC growth in response to hypoxia.
Figures


Similar articles
-
Restoration of Cullin3 gene expression enhances the improved effects of sonic hedgehog signaling activation for hypertension and attenuates the dysfunction of vascular smooth muscle cells.Biomed Eng Online. 2022 Jun 17;21(1):39. doi: 10.1186/s12938-022-01002-w. Biomed Eng Online. 2022. PMID: 35715796 Free PMC article.
-
Hedgehog-signaling is upregulated in non-producing human adrenal adenomas and antagonism of hedgehog-signaling inhibits proliferation of NCI-H295R cells and an immortalized primary human adrenal cell line.J Steroid Biochem Mol Biol. 2014 Jan;139:7-15. doi: 10.1016/j.jsbmb.2013.09.007. Epub 2013 Sep 21. J Steroid Biochem Mol Biol. 2014. PMID: 24063979
-
Hypoxia induced Sonic Hedgehog signaling regulates cancer stemness, epithelial-to-mesenchymal transition and invasion in cholangiocarcinoma.Exp Cell Res. 2019 Dec 15;385(2):111671. doi: 10.1016/j.yexcr.2019.111671. Epub 2019 Oct 18. Exp Cell Res. 2019. PMID: 31634481
-
Small-molecule modulators of the Sonic Hedgehog signaling pathway.Mol Biosyst. 2010 Jan;6(1):44-54. doi: 10.1039/b910196a. Epub 2009 Aug 27. Mol Biosyst. 2010. PMID: 20024066 Review.
-
Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review).Int J Mol Med. 2006 Dec;18(6):1019-23. Int J Mol Med. 2006. PMID: 17089004 Review.
Cited by
-
In smokers, Sonic hedgehog modulates pulmonary endothelial function through vascular endothelial growth factor.Respir Res. 2017 May 23;18(1):102. doi: 10.1186/s12931-017-0590-1. Respir Res. 2017. PMID: 28535764 Free PMC article.
-
The calcium binding protein S100β marks hedgehog-responsive resident vascular stem cells within vascular lesions.NPJ Regen Med. 2021 Mar 1;6(1):10. doi: 10.1038/s41536-021-00120-8. NPJ Regen Med. 2021. PMID: 33649337 Free PMC article.
-
Hedgehog and Resident Vascular Stem Cell Fate.Stem Cells Int. 2015;2015:468428. doi: 10.1155/2015/468428. Epub 2015 May 6. Stem Cells Int. 2015. PMID: 26064136 Free PMC article. Review.
-
Involvement of the mRNA binding protein CRD-BP in the regulation of metastatic melanoma cell proliferation and invasion by hypoxia.J Cell Sci. 2012 Dec 15;125(Pt 24):5950-4. doi: 10.1242/jcs.115204. Epub 2012 Oct 4. J Cell Sci. 2012. PMID: 23038779 Free PMC article.
-
A Two-Stage Whole-Genome Gene Expression Association Study of Young-Onset Hypertension in Han Chinese Population of Taiwan.Sci Rep. 2018 Jan 29;8(1):1800. doi: 10.1038/s41598-018-19520-w. Sci Rep. 2018. PMID: 29379041 Free PMC article.
References
-
- Mecham RP, Whitehouse LA, Wrenn DS, Parks WC, Griffin GL, Senior RM, Crouch EC, Stenmark KR, Voelkel NF. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science. 1987;237:423–426. - PubMed
-
- Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. - PubMed
-
- Orlandi A, Bochaton-Piallat ML, Gabbiani G, Spagnoli LG. Aging, smooth muscle cells and vascular pathobiology: implications for atherosclerosis. Atherosclerosis. 2006;188:221–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

