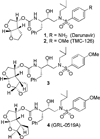
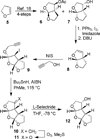
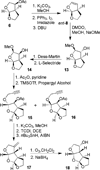
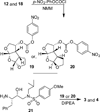
Probing multidrug-resistance and protein-ligand interactions with oxatricyclic designed ligands in HIV-1 protease inhibitors
- PMID: 20827746
- PMCID: PMC3523686
- DOI: 10.1002/cmdc.201000318
Probing multidrug-resistance and protein-ligand interactions with oxatricyclic designed ligands in HIV-1 protease inhibitors
Figures


Similar articles
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Design and synthesis of potent HIV-1 protease inhibitors incorporating hexahydrofuropyranol-derived high affinity P(2) ligands: structure-activity studies and biological evaluation.J Med Chem. 2011 Jan 27;54(2):622-34. doi: 10.1021/jm1012787. Epub 2010 Dec 31. J Med Chem. 2011. PMID: 21194227 Free PMC article.
-
Synthesis and biological evaluation of novel amprenavir-based P1-substituted bi-aryl derivatives as ultra-potent HIV-1 protease inhibitors.Bioorg Med Chem Lett. 2012 Mar 1;22(5):1976-9. doi: 10.1016/j.bmcl.2012.01.037. Epub 2012 Jan 21. Bioorg Med Chem Lett. 2012. PMID: 22306123
-
Substituted Bis-THF Protease Inhibitors with Improved Potency against Highly Resistant Mature HIV-1 Protease PR20.J Med Chem. 2015 Jun 25;58(12):5088-95. doi: 10.1021/acs.jmedchem.5b00474. Epub 2015 Jun 4. J Med Chem. 2015. PMID: 26010498 Free PMC article.
-
Resilience to resistance of HIV-1 protease inhibitors: profile of darunavir.AIDS Rev. 2008 Jul-Sep;10(3):131-42. AIDS Rev. 2008. PMID: 18820715 Free PMC article. Review.
Cited by
-
Phosphine-catalyzed divergent domino processes between γ-substituted allenoates and carbonyl-activated alkenes.Chem Sci. 2022 Feb 11;13(11):3161-3168. doi: 10.1039/d1sc06364b. eCollection 2022 Mar 16. Chem Sci. 2022. PMID: 35414887 Free PMC article.
-
The Pseudo-Symmetric N-benzyl Hydroxyethylamine Core in a New Series of Heteroarylcarboxyamide HIV-1 Pr Inhibitors: Synthesis, Molecular Modeling and Biological Evaluation.Biomolecules. 2021 Oct 26;11(11):1584. doi: 10.3390/biom11111584. Biomolecules. 2021. PMID: 34827582 Free PMC article.
-
Highly resistant HIV-1 proteases and strategies for their inhibition.Future Med Chem. 2015;7(8):1023-38. doi: 10.4155/fmc.15.44. Future Med Chem. 2015. PMID: 26062399 Free PMC article. Review.
-
Extreme multidrug resistant HIV-1 protease with 20 mutations is resistant to novel protease inhibitors with P1'-pyrrolidinone or P2-tris-tetrahydrofuran.J Med Chem. 2013 May 23;56(10):4017-27. doi: 10.1021/jm400231v. Epub 2013 May 1. J Med Chem. 2013. PMID: 23590295 Free PMC article.
-
Design, synthesis, and X-ray studies of potent HIV-1 protease inhibitors incorporating aminothiochromane and aminotetrahydronaphthalene carboxamide derivatives as the P2 ligands.Eur J Med Chem. 2018 Dec 5;160:171-182. doi: 10.1016/j.ejmech.2018.09.046. Epub 2018 Sep 18. Eur J Med Chem. 2018. PMID: 30340140 Free PMC article.
References
-
- Flexner C. N. Engl. J. Med. 1998;338:1281–1292. - PubMed
-
- Sepkowitz KA. N. Engl. J. Med. 2001;344:1764–1772. - PubMed
-
- Waters L, Nelson M. Int. J. Clin. Pract. 2007;61:983–990. - PubMed
-
- Pillay D, et al. AIDS. 2006;20:21–28. - PubMed
-
- Yerly S, Kaiser L, Race E, Bru JP, Clavel F, Perrin L. Lancet. 1999;354:729–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

